Abstract
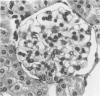
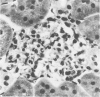
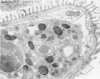
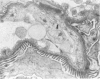
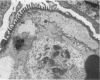
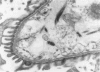
Tumor necrosis factor (TNF) is a polypeptide hormone produced by activated macrophages detectable in the circulation of experimental animals given endotoxin. Recent evidence strongly suggests that many of the deleterious effects of endotoxin in experimental animals are mediated by TNF. Because endotoxemia in experimental animals and humans is associated with glomerular damage the present investigation was designed to establish whether TNF directly induces glomerular functional and structural changes. Twenty-three rabbits were given human recombinant TNF at the doses of 0.08, 0.8, and 8.0 micrograms/kg/h as a continuous 5-hour intravenous infusion. Animals were killed at the end of the infusion. All rabbits given 0.8 and 8.0 micrograms/kg/h TNF developed anemia (Ht value decrease at 5 hours: 0.8 microgram/kg/h, 15%; 8.0 micrograms/kg/h, 16%); leukopenia (leukocyte count decrease at 5 hours: 0.8 micrograms/kg/h, 47%; 8.0 micrograms/kg/h, 59%); thrombocytopenia (platelet count decrease at 5 hours; 0.8 micrograms/kg/h, 45%; 8.0 micrograms/kg/h, 57%). Rabbits given 8.0 micrograms/kg/h also had renal failure (serum creatinine from 1.02 +/- 0.15 to 1.64 +/- 0.34 mg/dl). By light microscopy only occasional polymorphonuclear leukocytes in the glomerular capillaries were detectable in rabbits infused with 0.08 micrograms/kg/h TNF, whereas with 0.8 micrograms/kg/h TNF the presence of inflammatory cells in the glomerular capillaries was the prominent finding. With 8.0 micrograms/kg/h TNF beside leukocyte accumulation, fibrin was detected in the glomerular capillary lumens of two of eight animals. Electron microscopy found dose-dependent glomerular endothelial cell damage in animals given TNF with fibrinlike material in the capillary lumens. Glomerular changes induced by TNF were remarkably similar to those previously found in animals given endotoxin. Thus, TNF is likely to be the mediator of endotoxin-induced glomerular damage and can be regarded as a new mediator of macrophage-dependent damage in glomerulonephritis.
Full text
PDF



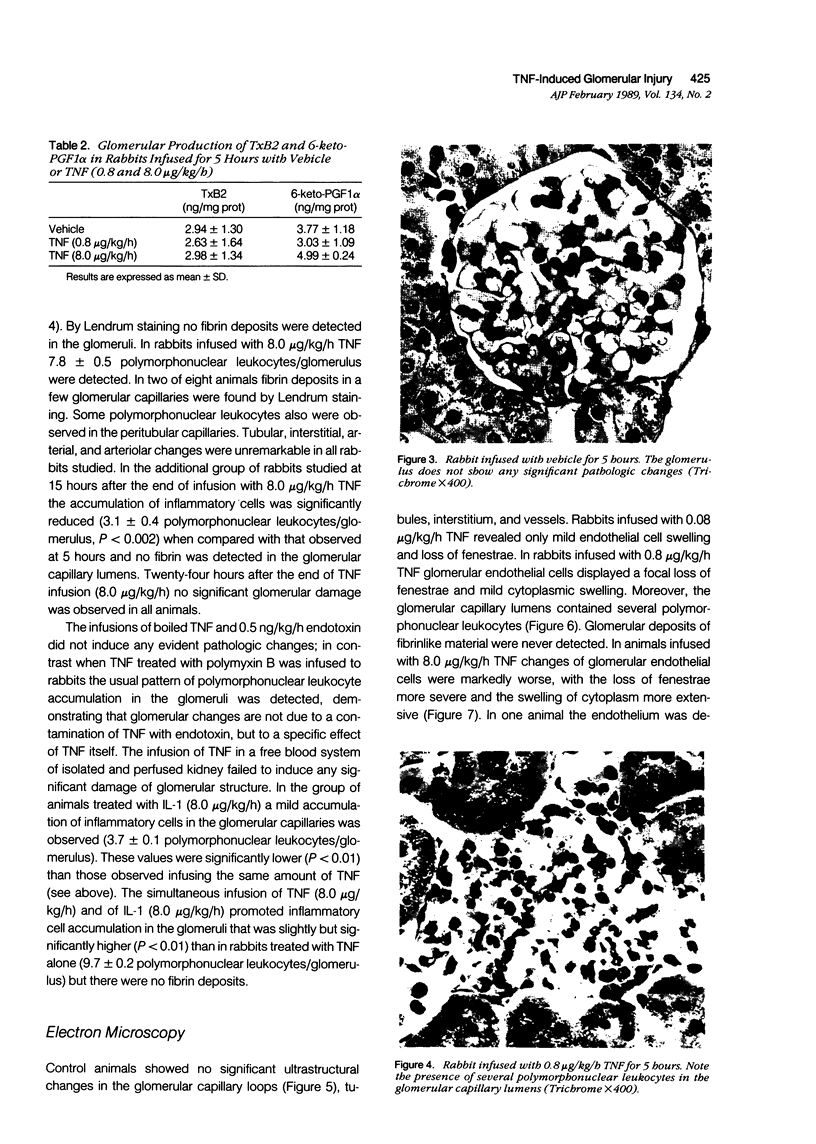

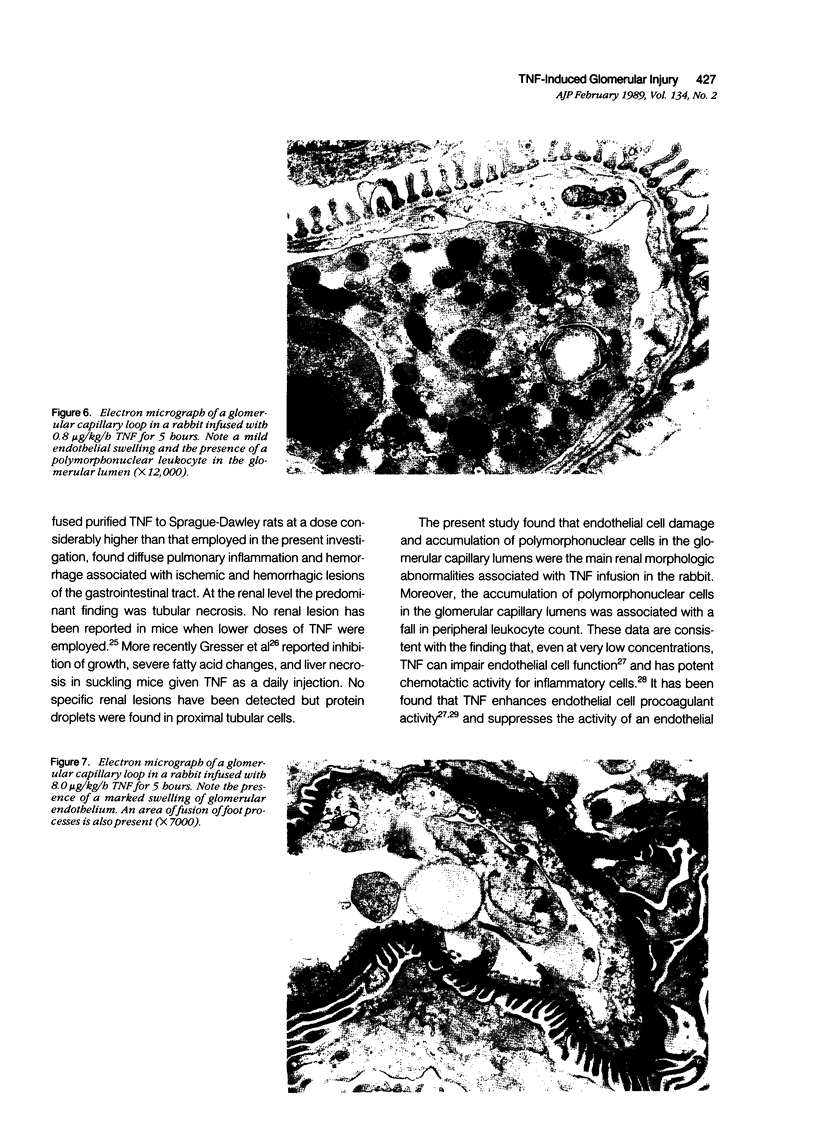
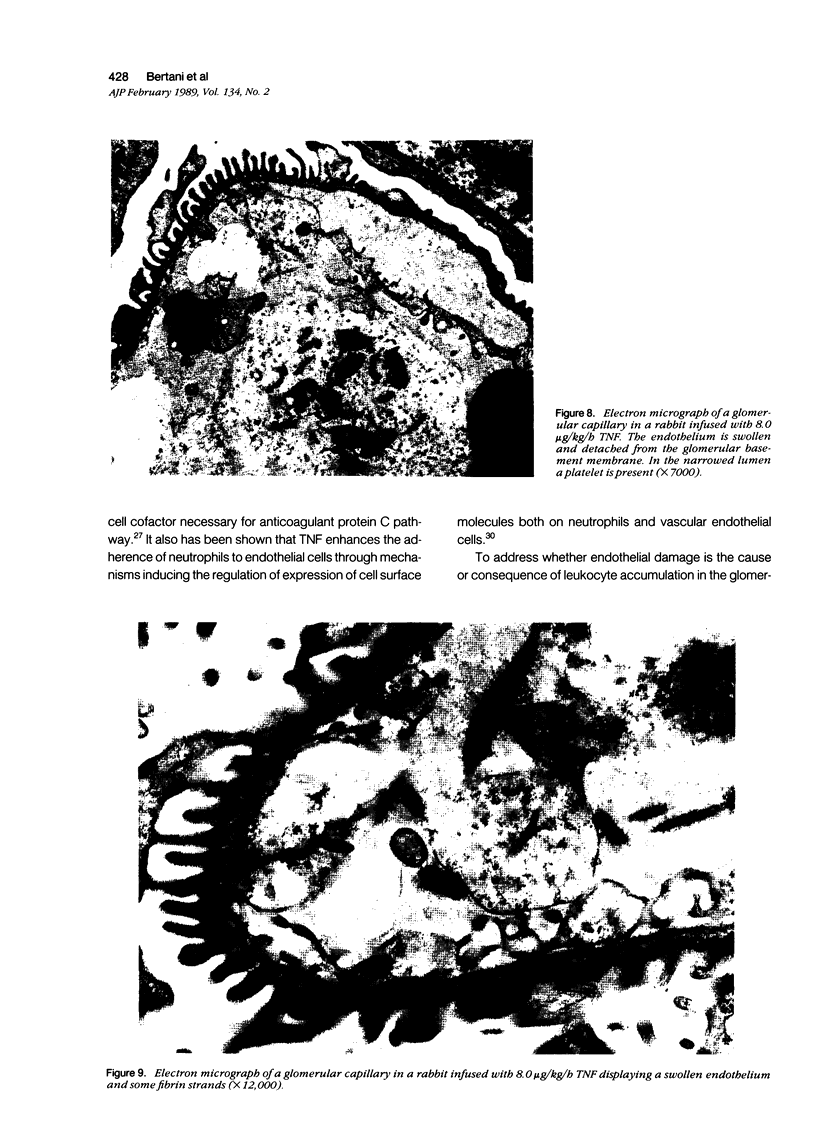


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe S., Gatanaga T., Yamazaki M., Soma G., Mizuno D. Purification of rabbit tumor necrosis factor. FEBS Lett. 1985 Jan 28;180(2):203–206. doi: 10.1016/0014-5793(85)81071-1. [DOI] [PubMed] [Google Scholar]
- Aggarwal B. B., Kohr W. J., Hass P. E., Moffat B., Spencer S. A., Henzel W. J., Bringman T. S., Nedwin G. E., Goeddel D. V., Harkins R. N. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985 Feb 25;260(4):2345–2354. [PubMed] [Google Scholar]
- BRUNSON J. G., GAMBLE C. N., THOMAS L. Morphologic changes in rabbits following the intravenous administration of meningococcal toxin. I. The effects produced in young and in mature animals by a single injection. Am J Pathol. 1955 May-Jun;31(3):489–499. [PMC free article] [PubMed] [Google Scholar]
- Badr K. F., Kelley V. E., Rennke H. G., Brenner B. M. Roles for thromboxane A2 and leukotrienes in endotoxin-induced acute renal failure. Kidney Int. 1986 Oct;30(4):474–480. doi: 10.1038/ki.1986.210. [DOI] [PubMed] [Google Scholar]
- Beutler B. A., Milsark I. W., Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985 Dec;135(6):3972–3977. [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Fiers W., Cotran R. S., Gimbrone M. A., Jr Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figari I. S., Mori N. A., Palladino M. A., Jr Regulation of neutrophil migration and superoxide production by recombinant tumor necrosis factors-alpha and -beta: comparison to recombinant interferon-gamma and interleukin-1 alpha. Blood. 1987 Oct;70(4):979–984. [PubMed] [Google Scholar]
- Gamble J. R., Harlan J. M., Klebanoff S. J., Vadas M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Woodrow D., Moss J., Maury C., Tavernier J., Fiers W. Toxic effects of recombinant tumor necrosis factor in suckling mice. Comparisons with interferon alpha/beta. Am J Pathol. 1987 Jul;128(1):13–18. [PMC free article] [PubMed] [Google Scholar]
- Gunther R. A., Rabinowitz L. Urea and renal concentrating ability in the rabbit. Kidney Int. 1980 Feb;17(2):205–222. doi: 10.1038/ki.1980.24. [DOI] [PubMed] [Google Scholar]
- Haranaka K., Carswell E. A., Williamson B. D., Prendergast J. S., Satomi N., Old L. J. Purification, characterization, and antitumor activity of nonrecombinant mouse tumor necrosis factor. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3949–3953. doi: 10.1073/pnas.83.11.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M., Ishibashi S., Ogawa H., Murase T., Takaku F., Shibata S. Cachectin/TNF as well as interleukin-1 induces prostacyclin synthesis in cultured vascular endothelial cells. Biochem Biophys Res Commun. 1986 Dec 15;141(2):482–487. doi: 10.1016/s0006-291x(86)80198-x. [DOI] [PubMed] [Google Scholar]
- Kettelhut I. C., Fiers W., Goldberg A. L. The toxic effects of tumor necrosis factor in vivo and their prevention by cyclooxygenase inhibitors. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4273–4277. doi: 10.1073/pnas.84.12.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Michalek S. M., Moore R. N., McGhee J. R., Rosenstreich D. L., Mergenhagen S. E. The primary role of lymphoreticular cells in the mediation of host responses to bacterial endotoxim. J Infect Dis. 1980 Jan;141(1):55–63. doi: 10.1093/infdis/141.1.55. [DOI] [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986 Mar 1;163(3):740–745. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima H., Weil M. H., Shubin H., Cavanilles J. Hemodynamic and metabolic studies on shock associated with gram negative bacteremia. Medicine (Baltimore) 1973 Jul;52(4):287–294. doi: 10.1097/00005792-197307000-00007. [DOI] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Raij L., Keane W. F., Michael A. F. Unilateral Shwartzman reaction: cortical necrosis in one kidney following in vivo perfusion with endotoxin. Kidney Int. 1977 Aug;12(2):91–95. doi: 10.1038/ki.1977.85. [DOI] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Remick D. G., Kunkel R. G., Larrick J. W., Kunkel S. L. Acute in vivo effects of human recombinant tumor necrosis factor. Lab Invest. 1987 Jun;56(6):583–590. [PubMed] [Google Scholar]
- Roblero J., Croxatto H., García R., Corthorn J., De Vito E. Kallikreinlike activity in perfusates and urine of isolated rat kidneys. Am J Physiol. 1976 Nov;231(5 Pt 1):1383–1389. doi: 10.1152/ajplegacy.1976.231.5.1383. [DOI] [PubMed] [Google Scholar]
- Shirai T., Yamaguchi H., Ito H., Todd C. W., Wallace R. B. Cloning and expression in Escherichia coli of the gene for human tumour necrosis factor. 1985 Feb 28-Mar 6Nature. 313(6005):803–806. doi: 10.1038/313803a0. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Finkelstein N., Aliminosa L., Crawford B., Graber M. THE RENAL CLEARANCES OF SUBSTITUTED HIPPURIC ACID DERIVATIVES AND OTHER AROMATIC ACIDS IN DOG AND MAN. J Clin Invest. 1945 May;24(3):388–404. doi: 10.1172/JCI101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Vio C. P., Churchill L., Rabito S. F., Terragno A., Carretero O. A., Terragno N. A. Renal kallikrein in venous effluent of filtering and non-filtering isolated kidneys. Adv Exp Med Biol. 1983;156(Pt B):897–905. [PubMed] [Google Scholar]
- Zoja C., Furci L., Ghilardi F., Zilio P., Benigni A., Remuzzi G. Cyclosporin-induced endothelial cell injury. Lab Invest. 1986 Oct;55(4):455–462. [PubMed] [Google Scholar]