Abstract
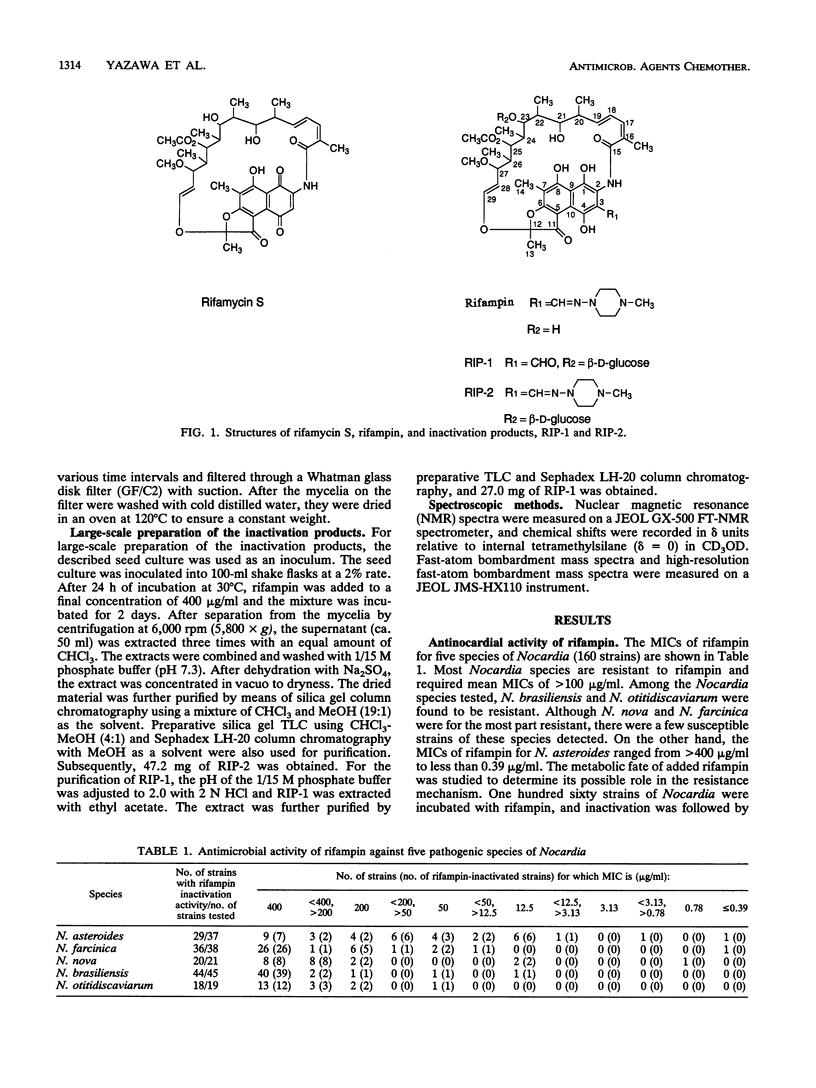
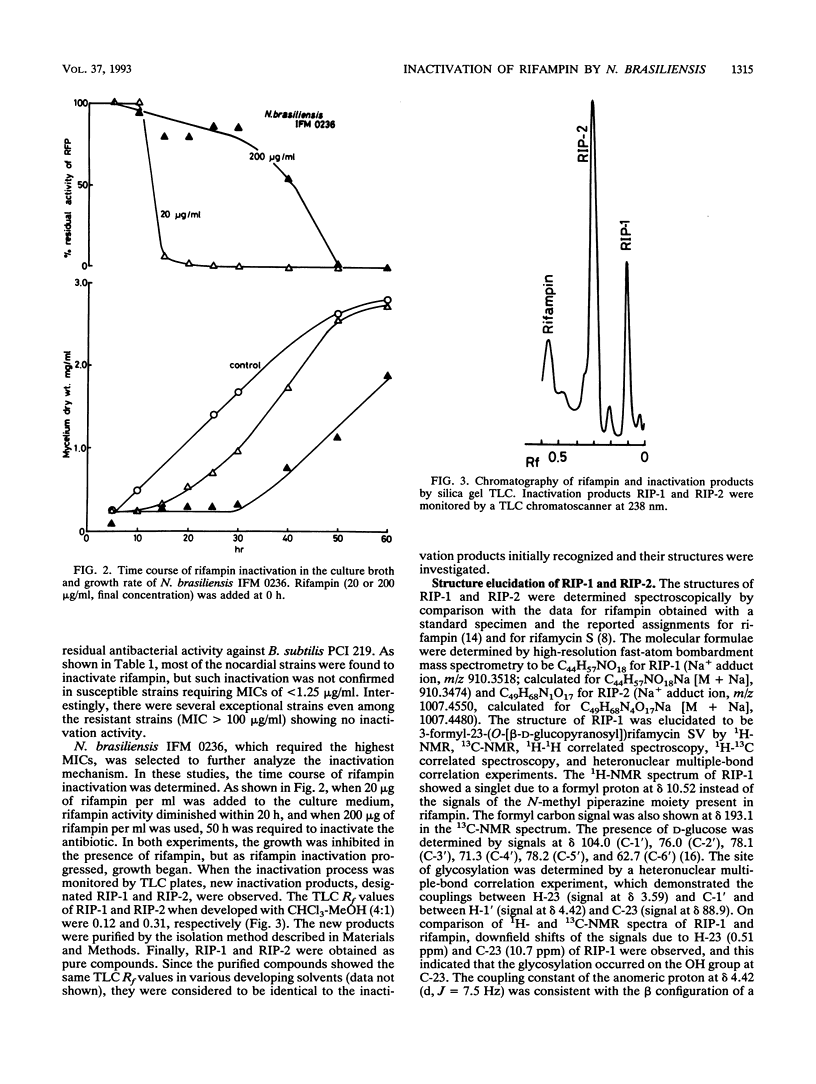
Rifampin was glycosylated by a pathogenic species of Nocardia, i.e., Nocardia brasiliensis. The structures of two glycosylated compounds (RIP-1 and RIP-2) isolated from the culture broth of the bacterium were determined to be 3-formyl-23-(O-[beta-D-glucopyranosyl])rifamycin SV and 23-(O-[beta-D-glucopyranosyl])rifampin, respectively. Both compounds lacked antimicrobial activity against other gram-positive bacteria as well as the Nocardia species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen S. J., Dabbs E. R. Cloning of nocardioform DNA conferring the ability to inactivate rifampicin. FEMS Microbiol Lett. 1991 Apr 15;63(2-3):247–250. doi: 10.1016/0378-1097(91)90093-p. [DOI] [PubMed] [Google Scholar]
- Arora S. K., Arjunan P. Molecular structure and conformation of rifamycin S, a potent inhibitor of DNA-dependent RNA polymerase. J Antibiot (Tokyo) 1992 Mar;45(3):428–431. doi: 10.7164/antibiotics.45.428. [DOI] [PubMed] [Google Scholar]
- Arora S. K., Main P. Correlation of structure and activity in ansamycins: molecular structure of cyclized rifamycin SV. J Antibiot (Tokyo) 1984 Feb;37(2):178–181. doi: 10.7164/antibiotics.37.178. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Aminoglycoside antibiotic-inactivating enzymes in actinomycetes similar to those present in clinical isolates of antibiotic-resistant bacteria. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2276–2280. doi: 10.1073/pnas.70.8.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brufani M., Cerrini S., Fedeli W., Vaciago A. Rifamycins: an insight into biological activity based on structural investigations. J Mol Biol. 1974 Aug 15;87(3):409–435. doi: 10.1016/0022-2836(74)90094-1. [DOI] [PubMed] [Google Scholar]
- Cavalleri B., Turconi M., Tamborini G., Occelli E., Cietto G., Pallanza R., Scotti R., Berti M., Romanó G., Parenti F. Synthesis and biological activity of some derivatives of rifamycin P. J Med Chem. 1990 May;33(5):1470–1476. doi: 10.1021/jm00167a029. [DOI] [PubMed] [Google Scholar]
- Fuhrer H. Zuordnung des 13C-NMR.-Spektrums von Rifamycin-S aufgrund der selektiven Protonen-Entkopplung. Helv Chim Acta. 1973 Jul;56(7):2377–2386. doi: 10.1002/hlca.19730560721. [DOI] [PubMed] [Google Scholar]
- Klemens S. P., Cynamon M. H. In vivo activities of newer rifamycin analogs against Mycobacterium avium infection. Antimicrob Agents Chemother. 1991 Oct;35(10):2026–2030. doi: 10.1128/aac.35.10.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M. S., Chirby D. G., Argoudelis A. D., Cialdella J. I., Coats J. H., Marshall V. P. Microbial glycosylation of erythromycin A. Antimicrob Agents Chemother. 1989 Dec;33(12):2089–2091. doi: 10.1128/aac.33.12.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisitsyn N. A., Sverdlov E. D., Moiseyeva E. P., Danilevskaya O. N., Nikiforov V. G. Mutation to rifampicin resistance at the beginning of the RNA polymerase beta subunit gene in Escherichia coli. Mol Gen Genet. 1984;196(1):173–174. doi: 10.1007/BF00334112. [DOI] [PubMed] [Google Scholar]
- Maggi N., Pasqualucci C. R., Ballotta R., Sensi P. Rifampicin: a new orally active rifamycin. Chemotherapy. 1966;11(5):285–292. doi: 10.1159/000220462. [DOI] [PubMed] [Google Scholar]
- Mikami Y., Takahashi K., Yazawa K., Arai T., Namikoshi M., Iwasaki S., Okuda S. Biosynthetic studies on saframycin A, a quinone antitumor antibiotic produced by Streptomyces lavendulae. J Biol Chem. 1985 Jan 10;260(1):344–348. [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. Actions of the rifamycins. Bacteriol Rev. 1971 Sep;35(3):290–309. doi: 10.1128/br.35.3.290-309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Nagata A., Ono Y., Suzuki Y., Yamanouchi T. Alteration of ribosomes and RNA polymerase in drug-resistant clinical isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1985 Jun;27(6):921–924. doi: 10.1128/aac.27.6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa K., Mikami Y., Maeda A., Kudo T., Suzuki K., Saito N., Kubo A. Inactivation of kanamycin A by phosphorylation in pathogenic Nocardia. Microbiol Immunol. 1991;35(1):39–48. doi: 10.1111/j.1348-0421.1991.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Yazawa K., Mikami Y., Ohashi S., Miyaji M., Ichihara Y., Nishimura C. In-vitro activity of new carbapenem antibiotics: comparative studies with meropenem, L-627 and imipenem against pathogenic Nocardia spp. J Antimicrob Chemother. 1992 Feb;29(2):169–172. doi: 10.1093/jac/29.2.169. [DOI] [PubMed] [Google Scholar]
- Yazawa K., Mikami Y., Uno J. In vitro susceptibility of Nocardia spp. to a new fluoroquinolone, tosufloxacin (T-3262). Antimicrob Agents Chemother. 1989 Dec;33(12):2140–2141. doi: 10.1128/aac.33.12.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]



