Abstract
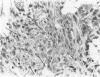
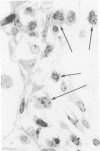
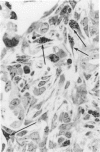
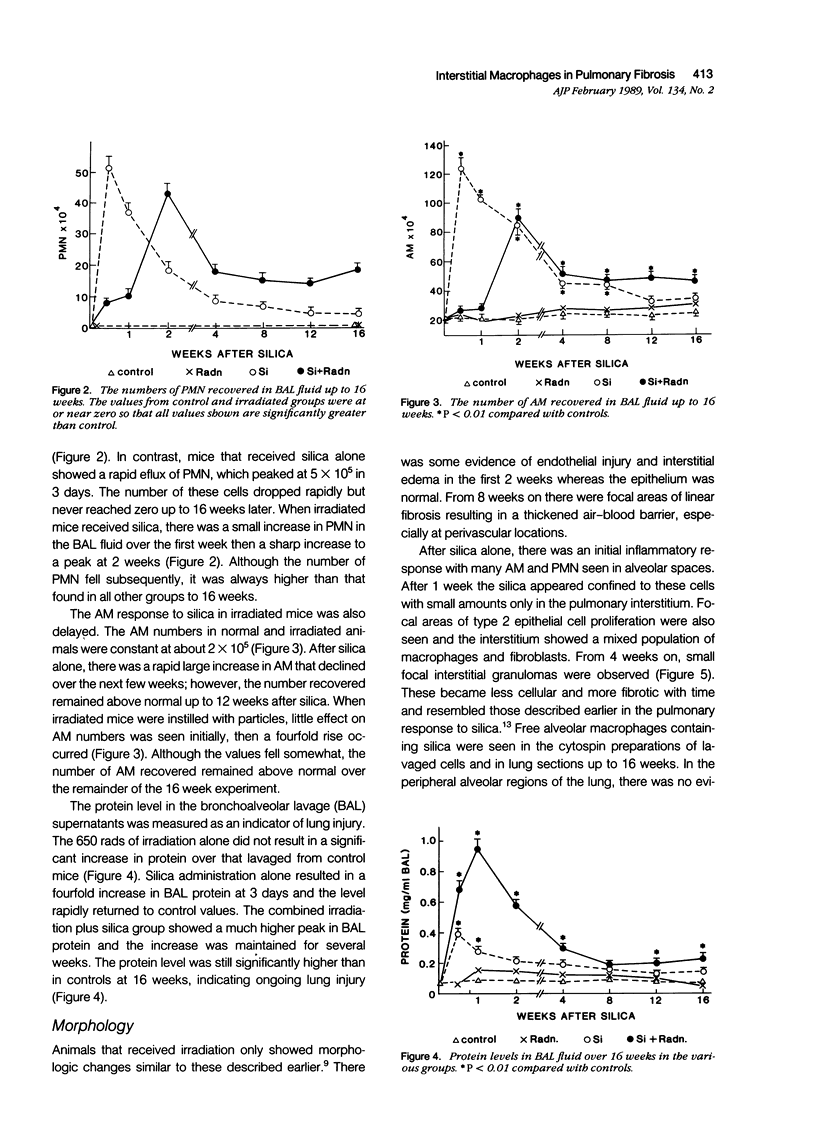
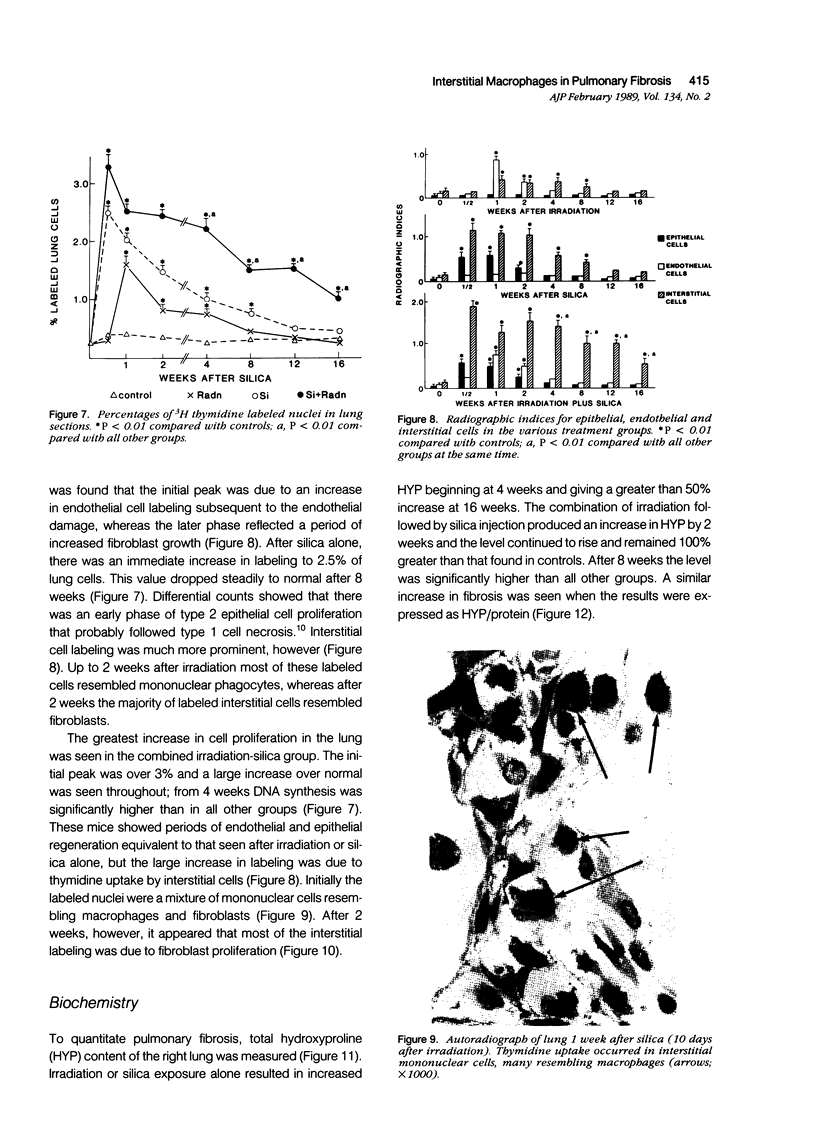
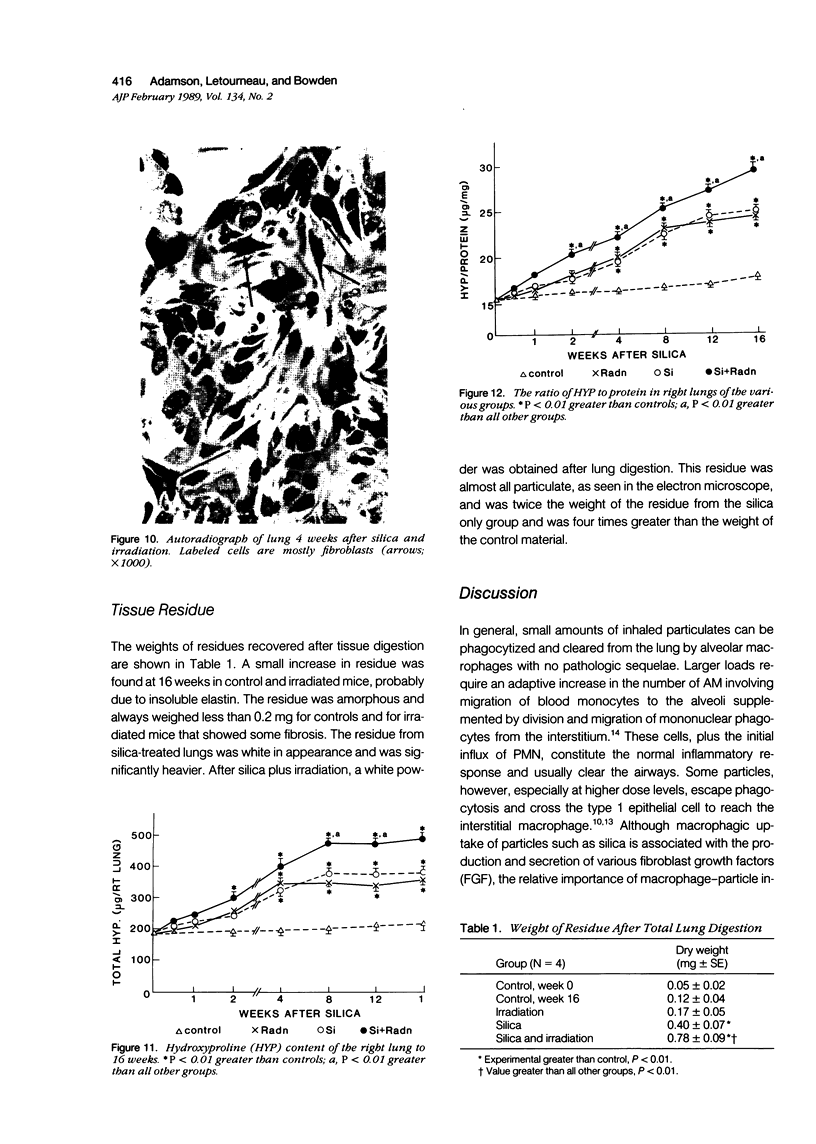
The role of interstitial vs. alveolar macrophages in the generation of pulmonary fibrosis after silica was examined. Using whole body irradiation to delay the inflammatory response and so retard particulate clearance, many more instilled silica particles reached the interstitial macrophages in the first 2 weeks than after silica alone. This was followed by greatly increased fibroblast proliferation and deposition of collagen in the irradiation plus silica group, which developed large interstitial granulomas at the sites of silica retention. Although alveolar macrophages containing silica were seen in both silica groups, more interstitial particles were observed after combined irradiation and silica, significantly more silica was recovered in a residue from the lungs at 16 weeks, and pulmonary fibrosis at 8-16 weeks was greater than in all other groups. The results indicate that increased fibroblast growth and collagen synthesis in vivo are associated with phagocytosis of silica by interstitial macrophages rather than by free alveolar macrophages. It is suggested that transfer of a macrophages-derived growth factor to fibroblasts is more efficient when it occurs within the pulmonary interstitium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalto M., Potila M., Kulonen E. The effect of silica-treated macrophages on the synthesis of collagen and other proteins in vitro. Exp Cell Res. 1976 Jan;97:193–202. doi: 10.1016/0014-4827(76)90668-6. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. Effects of irradiation on macrophagic response and transport of particles across the alveolar epithelium. Am J Pathol. 1982 Jan;106(1):40–46. [PMC free article] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. Endothelial injury and repair in radiation-induced pulmonary fibrosis. Am J Pathol. 1983 Aug;112(2):224–230. [PMC free article] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. Response of mouse lung to crocidolite asbestos. 1. Minimal fibrotic reaction to short fibres. J Pathol. 1987 Jun;152(2):99–107. doi: 10.1002/path.1711520206. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. Response of mouse lung to crocidolite asbestos. 2. Pulmonary fibrosis after long fibres. J Pathol. 1987 Jun;152(2):109–117. doi: 10.1002/path.1711520207. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. Role of polymorphonuclear leukocytes in silica-induced pulmonary fibrosis. Am J Pathol. 1984 Oct;117(1):37–43. [PMC free article] [PubMed] [Google Scholar]
- Bateman E. D., Emerson R. J., Cole P. J. A study of macrophage-mediated initiation of fibrosis by asbestos and silica using a diffusion chamber technique. Br J Exp Pathol. 1982 Aug;63(4):414–425. [PMC free article] [PubMed] [Google Scholar]
- Bitterman P. B., Adelberg S., Crystal R. G. Mechanisms of pulmonary fibrosis. Spontaneous release of the alveolar macrophage-derived growth factor in the interstitial lung disorders. J Clin Invest. 1983 Nov;72(5):1801–1813. doi: 10.1172/JCI111140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. Adaptive responses of the pulmonary macrophagic system to carbon. I. Kinetic studies. Lab Invest. 1978 Apr;38(4):422–429. [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. The role of cell injury and the continuing inflammatory response in the generation of silicotic pulmonary fibrosis. J Pathol. 1984 Nov;144(3):149–161. doi: 10.1002/path.1711440302. [DOI] [PubMed] [Google Scholar]
- Brain J. D. Lung macrophages: how many kinds are there? What do they do? Am Rev Respir Dis. 1988 Mar;137(3):507–509. doi: 10.1164/ajrccm/137.3.507. [DOI] [PubMed] [Google Scholar]
- Davis G. S. Pathogenesis of silicosis: current concepts and hypotheses. Lung. 1986;164(3):139–154. doi: 10.1007/BF02713638. [DOI] [PubMed] [Google Scholar]
- Dethloff L. A., Lehnert B. E. Pulmonary interstitial macrophages: isolation and flow cytometric comparisons with alveolar macrophages and blood monocytes. J Leukoc Biol. 1988 Jan;43(1):80–90. doi: 10.1002/jlb.43.1.80. [DOI] [PubMed] [Google Scholar]
- Goldstein R. H., Fine A. Fibrotic reactions in the lung: the activation of the lung fibroblast. Exp Lung Res. 1986;11(4):245–261. doi: 10.3109/01902148609062828. [DOI] [PubMed] [Google Scholar]
- Heppleston A. G. Pulmonary toxicology of silica, coal and asbestos. Environ Health Perspect. 1984 Apr;55:111–127. doi: 10.1289/ehp.8455111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lugano E. M., Dauber J. H., Elias J. A., Bashey R. I., Jimenez S. A., Daniele R. P. The regulation of lung fibroblast proliferation by alveolar macrophages in experimental silicosis. Am Rev Respir Dis. 1984 May;129(5):767–771. doi: 10.1164/arrd.1984.129.5.767. [DOI] [PubMed] [Google Scholar]