Abstract
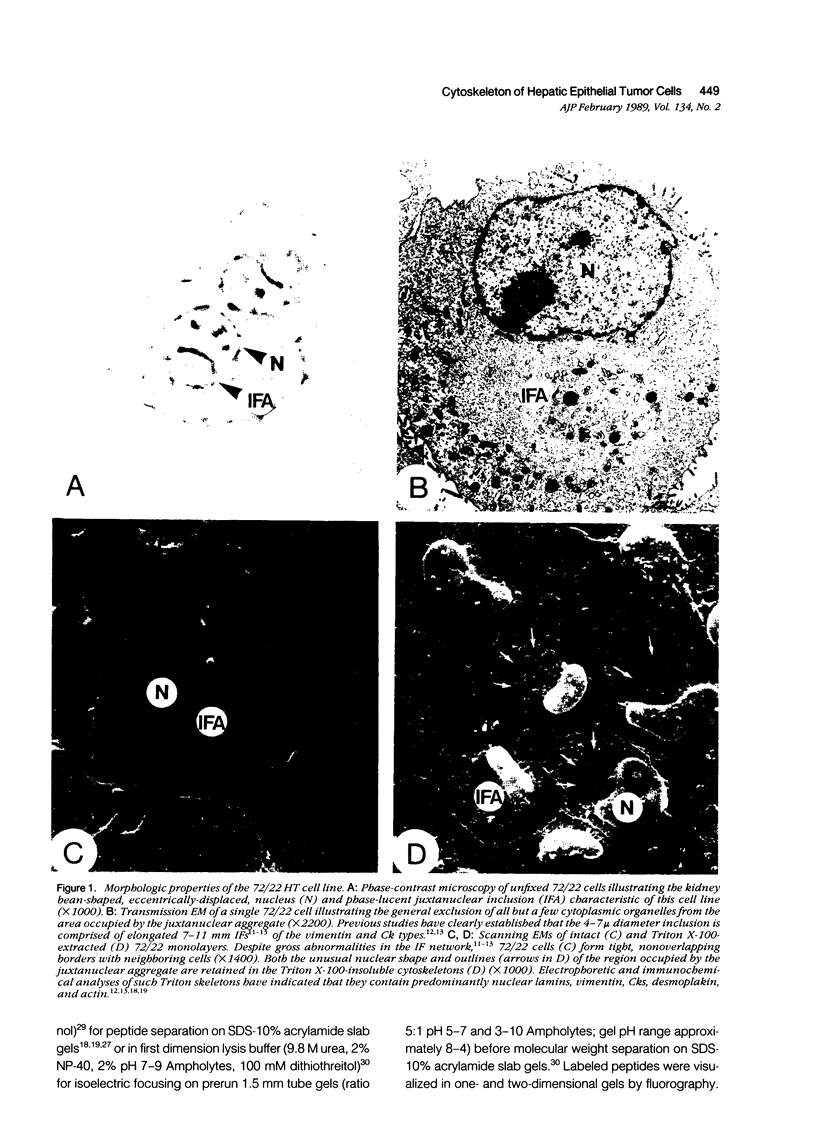
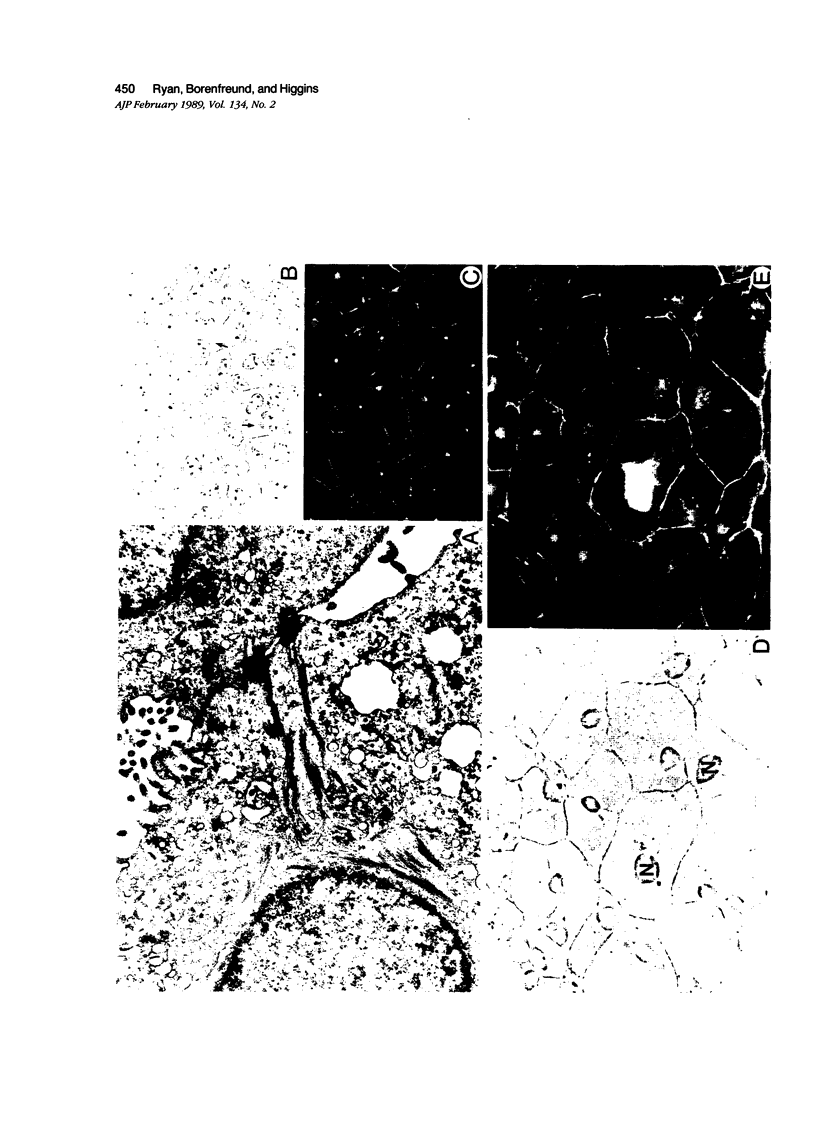
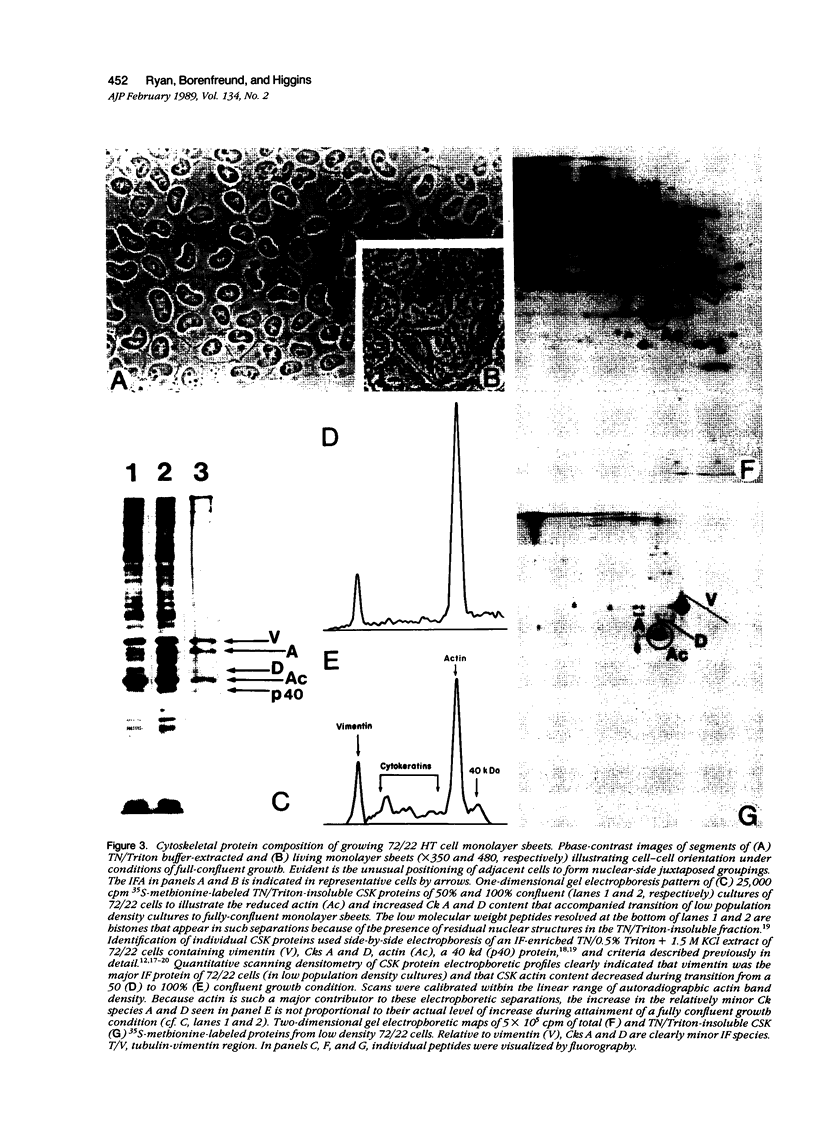
Established 72/22 rat hepatic tumor cells, which bear well-characterized cytoplasmic abnormalities of intermediate filament (IF) network organization, form monolayer "sheets" of tightly juxtaposed epithelial cells at high culture population densities. The distribution of desmosomal complexes and their affiliated tonofilaments, as well as the regulation of cytokeratin/vimentin IF and actin microfilament contents were assessed during construction of this in vitro "epithelium." 72/22 cells formed desmosomal junctions throughout the length of the cellular perimeter. Compared with low population density cultures, fully confluent sheets of 72/22 cells exhibited a down-regulated cytoskeletal actin content and increased level of cytokeratin synthesis. Despite gross IF cytoarchitectural abnormalities, 72/22 cells normally modulated the content of specific structural elements within both the IF and microfilament networks in response to increasing cell-cell contact. Furthermore, these data support the concept that neither the structural integrity nor the topographic distribution of the desmosome array are dependent on tonofilament anchorage or IF scaffold organization.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Ze'ev A. Cell-cell interaction and cell configuration related control of cytokeratins and vimentin expression in epithelial cells and in fibroblasts. Ann N Y Acad Sci. 1985;455:597–613. doi: 10.1111/j.1749-6632.1985.tb50439.x. [DOI] [PubMed] [Google Scholar]
- Berger G., Berger F., Bejui F., Bouvier R., Rochet M., Feroldi J. Bronchial carcinoid with fibrillary inclusions related to cytokeratins: an immunohistochemical and ultrastructural study with subsequent investigation of 12 foregut APUDomas. Histopathology. 1984 Mar;8(2):245–257. doi: 10.1111/j.1365-2559.1984.tb02339.x. [DOI] [PubMed] [Google Scholar]
- Borenfreund E., Higgins P. J., Bendich A. In vivo initiated rat liver carcinogenesis studied in vitro; formation of alcoholic hyaline-type bodies. Cancer Lett. 1977 Sep;3(3-4):145–150. doi: 10.1016/s0304-3835(77)95126-6. [DOI] [PubMed] [Google Scholar]
- Borenfreund E., Higgins P. J., Peterson E. Intermediate-sized filaments in cultured rat liver tumor cells with Mallory body-like cytoplasm abnormalities. J Natl Cancer Inst. 1980 Feb;64(2):323–333. doi: 10.1093/jnci/64.2.323. [DOI] [PubMed] [Google Scholar]
- Borenfreund E., Higgins P. J., Steinglass M., Bendich A. Carcinogen-induced abnormalities in rat liver cells and their modification by chemical agents. Cancer Res. 1979 Mar;39(3):800–807. [PubMed] [Google Scholar]
- Borenfreund E., Schmid E., Bendich A., Franke W. W. Constitutive aggregates of intermediate-sized filaments of the vimentin and cytokeratin type in cultured hepatoma cells and their dispersal by butyrate. Exp Cell Res. 1980 May;127(1):215–235. doi: 10.1016/0014-4827(80)90428-0. [DOI] [PubMed] [Google Scholar]
- Capetanaki Y. G., Flytzanis C. N., Alonso A. Repression of the albumin gene in Novikoff hepatoma cells. Mol Cell Biol. 1982 Mar;2(3):258–266. doi: 10.1128/mcb.2.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin P., Kapprell H. P., Franke W. W. The complement of desmosomal plaque proteins in different cell types. J Cell Biol. 1985 Oct;101(4):1442–1454. doi: 10.1083/jcb.101.4.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernobilsky B., Moll R., Leppien G., Schweikhart G., Franke W. W. Desmosomal plaque-associated vimentin filaments in human ovarian granulosa cell tumors of various histologic patterns. Am J Pathol. 1987 Mar;126(3):476–486. [PMC free article] [PubMed] [Google Scholar]
- Denk H., Eckerstorfer R. Colchicine-induced Mallory body formation in the mouse. Lab Invest. 1977 Jun;36(6):563–565. [PubMed] [Google Scholar]
- Denk H., Eckerstorfer R., Gschnait F., Konrad K., Wolff K. Experimental induction of hepatocellular hyalin (Mallory bodies) in mice by griseofulvin treatment. 1. Light microscopic observation. Lab Invest. 1976 Oct;35(4):377–382. [PubMed] [Google Scholar]
- Denk H., Franke W. W., Dragosics B., Zeiler I. Pathology of cytoskeleton of liver cells: demonstration of mallory bodies (alcoholic hyalin) in murine and human hepatocytes by immunofluorescence microscopy using antibodies to cytokeratin polypeptides from hepatocytes. Hepatology. 1981 Jan-Feb;1(1):9–20. doi: 10.1002/hep.1840010103. [DOI] [PubMed] [Google Scholar]
- Denk H., Franke W. W., Eckerstorfer R., Schmid E., Kerjaschki D. Formation and involution of Mallory bodies ("alcoholic hyalin") in murine and human liver revealed by immunofluorescence microscopy with antibodies to prekeratin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4112–4116. doi: 10.1073/pnas.76.8.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk H., Franke W. W., Kerjaschki D., Eckerstorfer R. Mallory bodies in experimental animals and man. Int Rev Exp Pathol. 1979;20:77–121. [PubMed] [Google Scholar]
- Denk H., Franke W. W. Rearrangement of the hepatocyte cytoskeleton after toxic damage: involution, dispersal and peripheral accumulation of Mallory body material after drug withdrawal. Eur J Cell Biol. 1981 Feb;23(2):241–249. [PubMed] [Google Scholar]
- Denk H., Krepler R., Lackinger E., Artlieb U., Franke W. W. Biochemical and immunocytochemical analysis of the intermediate filament cytoskeleton in human hepatocellular carcinomas and in hepatic neoplastic nodules of mice. Lab Invest. 1982 Jun;46(6):584–596. [PubMed] [Google Scholar]
- Denk H., Lackinger E., Cowin P., Franke W. W. Maintenance of desmosomes in mouse hepatocytes after drug-induced rearrangement of cytokeratin filament material. Demonstration of independence of desmosomes and intermediate-sized filaments. Exp Cell Res. 1985 Nov;161(1):161–171. doi: 10.1016/0014-4827(85)90500-2. [DOI] [PubMed] [Google Scholar]
- Erlandson R. A., Cardon-Cardo C., Higgins P. J. Histogenesis of benign pleomorphic adenoma (mixed tumor) of the major salivary glands. An ultrastructural and immunohistochemical study. Am J Surg Pathol. 1984 Nov;8(11):803–820. doi: 10.1097/00000478-198411000-00001. [DOI] [PubMed] [Google Scholar]
- Fischer H. P., Altmannsberger M., Weber K., Osborn M. Keratin polypeptides in malignant epithelial liver tumors. Differential diagnostic and histogenetic aspects. Am J Pathol. 1987 Jun;127(3):530–537. [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Denk H., Kalt R., Schmid E. Biochemical and immunological identification of cytokeratin proteins present in hepatocytes of mammalian liver tissue. Exp Cell Res. 1981 Feb;131(2):299–318. doi: 10.1016/0014-4827(81)90234-2. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Denk H., Schmid E., Osborn M., Weber K. Ultrastructural, biochemical, and immunologic characterization of Mallory bodies in livers of griseofulvin-treated mice. Fimbriated rods of filaments containing prekeratin-like polypeptides. Lab Invest. 1979 Feb;40(2):207–220. [PubMed] [Google Scholar]
- Franke W. W., Mayer D., Schmid E., Denk H., Borenfreund E. Differences of expression of cytoskeletal proteins in cultured rat hepatocytes and hepatoma cells. Exp Cell Res. 1981 Aug;134(2):345–365. doi: 10.1016/0014-4827(81)90435-3. [DOI] [PubMed] [Google Scholar]
- French S. W., Katsuma Y., Ray M. B., Swierenga S. H. Cytoskeletal pathology induced by ethanol. Ann N Y Acad Sci. 1987;492:262–276. doi: 10.1111/j.1749-6632.1987.tb48680.x. [DOI] [PubMed] [Google Scholar]
- Goldman R., Goldman A., Green K., Jones J., Lieska N., Yang H. Y. Intermediate filaments: possible functions as cytoskeletal connecting links between the nucleus and the cell surface. Ann N Y Acad Sci. 1985;455:1–17. doi: 10.1111/j.1749-6632.1985.tb50400.x. [DOI] [PubMed] [Google Scholar]
- Hazan R., Denk H., Franke W. W., Lackinger E., Schiller D. L. Change of cytokeratin organization during development of Mallory bodies as revealed by a monoclonal antibody. Lab Invest. 1986 May;54(5):543–553. [PubMed] [Google Scholar]
- Higgins P. J. Characterization of the growth inhibited substate induced in murine hepatic tumor cells during in vitro exposure to dimethylsulfoxide. Int J Cancer. 1986 Dec 15;38(6):889–899. doi: 10.1002/ijc.2910380617. [DOI] [PubMed] [Google Scholar]
- Higgins P. J., Lipkin G., Rosenberg M., Ryan M. P. Contact-inhibitory factor induces alterations in the distribution and content of specific cytoskeletal elements in an established line of rat hepatic tumor cells. Int J Cancer. 1987 Dec 15;40(6):792–801. doi: 10.1002/ijc.2910400615. [DOI] [PubMed] [Google Scholar]
- Higgins P. J., Silverstone A. E., Bueti C., Pizzi V. F., Melamed M. R., Lipkin M., Traganos F. Expression of murine gamma fetal antigen in adult hematopoietic tissue and during induced differentiation of Friend erythroleukemia cells. J Natl Cancer Inst. 1986 May;76(5):885–893. [PubMed] [Google Scholar]
- Higgins P. J., Staiano-Coico L., Evenson D. P., Melamed M. R. Characterization and carcinogen sensitivity of an established endothelial-like cell line derived from adult rat liver tissue. Oncology. 1984;41(5):331–337. doi: 10.1159/000225849. [DOI] [PubMed] [Google Scholar]
- Horvath E., Kovacs K. Morphogenesis and significance of fibrous bodies in human pituitary adenomas. Virchows Arch B Cell Pathol. 1978 Mar 2;27(1):69–78. doi: 10.1007/BF02888984. [DOI] [PubMed] [Google Scholar]
- Höfler H., Kerl H., Lackinger E., Helleis G., Denk H. The intermediate filament cytoskeleton of cutaneous neuroendocrine carcinoma (Merkel cell tumour). Immunohistochemical and biochemical analyses. Virchows Arch A Pathol Anat Histopathol. 1985;406(3):339–350. doi: 10.1007/BF00704303. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Moll R., Osborn M., Hartschuh W., Moll I., Mahrle G., Weber K. Variability of expression and arrangement of cytokeratin and neurofilaments in cutaneous neuroendocrine carcinomas (Merkel cell tumors): immunocytochemical and biochemical analysis of twelve cases. Ultrastruct Pathol. 1986;10(6):473–495. doi: 10.3109/01913128609007206. [DOI] [PubMed] [Google Scholar]
- Neumann P. E., Horoupian D. S., Goldman J. E., Hess M. A. Cytoplasmic filaments of Crooke's hyaline change belong to the cytokeratin class. An immunocytochemical and ultrastructural study. Am J Pathol. 1984 Aug;116(2):214–222. [PMC free article] [PubMed] [Google Scholar]
- Ryan M. P., Borenfreund E., Higgins P. J. Butyrate-induced cytoarchitectural reorganization of Mallory body-containing rat hepatic tumor cells. J Natl Cancer Inst. 1987 Sep;79(3):555–567. [PubMed] [Google Scholar]
- Ryan M. P., Higgins P. J. Discrimination between the nuclear lamin and intermediate filament (cytokeratin/vimentin) proteins of rat hepatic tumor cells by differential solubility and electrophoretic criteria. Int J Biochem. 1987;19(12):1187–1192. doi: 10.1016/0020-711x(87)90101-7. [DOI] [PubMed] [Google Scholar]
- Sibley R. K., Dehner L. P., Rosai J. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. I. A clinicopathologic and ultrastructural study of 43 cases. Am J Surg Pathol. 1985 Feb;9(2):95–108. doi: 10.1097/00000478-198502000-00004. [DOI] [PubMed] [Google Scholar]
- Tang C. K., Toker C. Trabecular carcinoma of the skin: an ultrastructural study. Cancer. 1978 Nov;42(5):2311–2321. doi: 10.1002/1097-0142(197811)42:5<2311::aid-cncr2820420531>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Tinberg H. M. Intermediate filaments: analysis of filamentous aggregates induced by griseofulvin, an antitubulin agent. Biochem Biophys Res Commun. 1981 Mar 31;99(2):458–465. doi: 10.1016/0006-291x(81)91767-8. [DOI] [PubMed] [Google Scholar]
- Venetianer A., Schiller D. L., Magin T., Franke W. W. Cessation of cytokeratin expression in a rat hepatoma cell line lacking differentiated functions. Nature. 1983 Oct 20;305(5936):730–733. doi: 10.1038/305730a0. [DOI] [PubMed] [Google Scholar]
- Virtanen I., Närvänen O., Lehto V. P. Differential immunoreactivity and Ca2+-dependent degradation of vimentin in human fibroblasts and fibrosarcoma cells. Int J Cancer. 1988 Aug 15;42(2):256–260. doi: 10.1002/ijc.2910420219. [DOI] [PubMed] [Google Scholar]
- Vogel A. M., Gown A. M., Caughlan J., Haas J. E., Beckwith J. B. Rhabdoid tumors of the kidney contain mesenchymal specific and epithelial specific intermediate filament proteins. Lab Invest. 1984 Feb;50(2):232–238. [PubMed] [Google Scholar]
- Wahrman M. Z., Gagnier S. E., Kobrin D. R., Higgins P. J., Augenlicht L. H. Cellular and molecular changes in 3T3 cells transformed spontaneously or by DNA transfection. Tumour Biol. 1985;6(1):41–56. [PubMed] [Google Scholar]
- Yokoo H., Minick O. T., Batti F., Kent G. Morphologic variants of alcoholic hyalin. Am J Pathol. 1972 Oct;69(1):25–40. [PMC free article] [PubMed] [Google Scholar]