Abstract
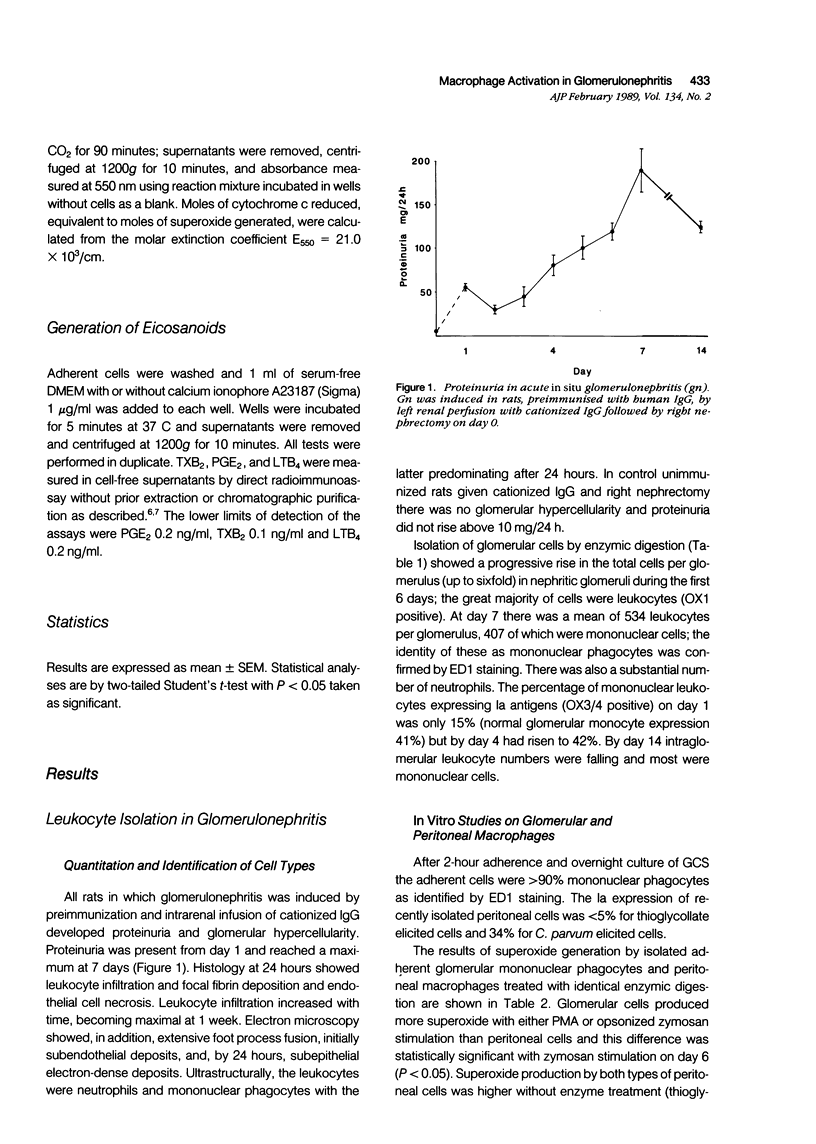
Macrophage infiltration is important in the pathogenesis of acute proliferative glomerulonephritis (gn). The state of activation of macrophages during gn may be central to their role in injury. To study this, a method for extracting macrophages from nephritic glomeruli in active in situ gn was developed. MHC Class II (Ia) antigen expression, superoxide (O2-) generation, and eicosanoid synthesis were compared with thioglycollate elicited peritoneal macrophages (TEM). At the height of inflammation there were 407 +/- 83 macrophages/glomerulus. Compared with TEM, Ia expression, and in vitro production of O2- were enhanced. Synthesis of prostaglandin E2 was greatly reduced (day 6 gn, 62 +/- 10 ng/mg; TEM 663 +/- 128 ng/mg cell protein). Thromboxane synthesis was relatively conserved (day 6 gn, 109 +/- 28 ng/mg; TEM 201 +/- 53 ng/mg). Leukotriene B4 (LTB4) was undetectable (day 6 gn, less than 13 ng/mg; TEM 119 +/- 56 ng/mg). This large influx of activated macrophages in glomeruli may be fundamental to pathogenesis of glomerular inflammation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. A., Cohn Z. A. Bacterial lipopolysaccharides modify signal transduction in the arachidonic acid cascade in macrophages. Ciba Found Symp. 1986;118:196–210. doi: 10.1002/9780470720998.ch13. [DOI] [PubMed] [Google Scholar]
- Baud L., Hagege J., Sraer J., Rondeau E., Perez J., Ardaillou R. Reactive oxygen production by cultured rat glomerular mesangial cells during phagocytosis is associated with stimulation of lipoxygenase activity. J Exp Med. 1983 Dec 1;158(6):1836–1852. doi: 10.1084/jem.158.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomford R., Christie G. H. Mechanisms of macrophage activation by corynebacterium parvum. II. In vivo experiments. Cell Immunol. 1975 May;17(1):150–155. doi: 10.1016/s0008-8749(75)80015-3. [DOI] [PubMed] [Google Scholar]
- Boraschi D., Censini S., Bartalini M., Ghiara P., Di Simplicio P., Tagliabue A. Interferons inhibit LTC4 production in murine macrophages. J Immunol. 1987 Jun 15;138(12):4341–4346. [PubMed] [Google Scholar]
- Boyce N. W., Holdsworth S. R. Hydroxyl radical mediation of immune renal injury by desferrioxamine. Kidney Int. 1986 Dec;30(6):813–817. doi: 10.1038/ki.1986.260. [DOI] [PubMed] [Google Scholar]
- Cattell V., Cook H. T., Smith J., Salmon J. A., Moncada S. Leukotriene B4 production in normal rat glomeruli. Nephrol Dial Transplant. 1987;2(3):154–157. [PubMed] [Google Scholar]
- Chensue S. W., Ellul D. A., Spengler M., Higashi G. I., Kunkel S. L. Dynamics of arachidonic acid metabolism in macrophages from delayed-type hypersensitivity (Schistosoma mansoni egg) and foreign-body-type granulomas. J Leukoc Biol. 1985 Dec;38(6):671–686. doi: 10.1002/jlb.38.6.671. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Kunkel S. L. Arachidonic acid metabolism and macrophage activation. Clin Lab Med. 1983 Dec;3(4):677–694. [PubMed] [Google Scholar]
- Cook H. T., Cattell V., Smith J., Salmon J. A., Moncada S. Effect of a thromboxane synthetase inhibitor on eicosanoid synthesis and glomerular injury during acute unilateral glomerulonephritis in the rat. Clin Nephrol. 1986 Oct;26(4):195–202. [PubMed] [Google Scholar]
- Cook H. T., Smith J., Cattell V. Isolation and characterization of inflammatory leukocytes from glomeruli in an in situ model of glomerulonephritis in the rat. Am J Pathol. 1987 Jan;126(1):126–136. [PMC free article] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Gurner A., Smith J., Cattell V. In vivo induction of Ia antigen in resident leukocytes in the normal rat renal glomerulus. Lab Invest. 1986 Nov;55(5):546–550. [PubMed] [Google Scholar]
- Hamilton T. A., Becton D. L., Somers S. D., Gray P. W., Adams D. O. Interferon-gamma modulates protein kinase C activity in murine peritoneal macrophages. J Biol Chem. 1985 Feb 10;260(3):1378–1381. [PubMed] [Google Scholar]
- Humes J. L., Burger S., Galavage M., Kuehl F. A., Jr, Wightman P. D., Dahlgren M. E., Davies P., Bonney R. J. The diminished production of arachidonic acid oxygenation products by elicited mouse peritoneal macrophages: possible mechanisms. J Immunol. 1980 May;124(5):2110–2116. [PubMed] [Google Scholar]
- Johnson R. J., Couser W. G., Chi E. Y., Adler S., Klebanoff S. J. New mechanism for glomerular injury. Myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest. 1987 May;79(5):1379–1387. doi: 10.1172/JCI112965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Kitagawa S. Molecular basis for the enhanced respiratory burst of activated macrophages. Fed Proc. 1985 Nov;44(14):2927–2932. [PubMed] [Google Scholar]
- Kunkel S. L., Chensue S. W., Phan S. H. Prostaglandins as endogenous mediators of interleukin 1 production. J Immunol. 1986 Jan;136(1):186–192. [PubMed] [Google Scholar]
- Kunkel S. L., Zanetti M., Sapin C. Suppression of nephrotoxic serum nephritis in rats by prostaglandin E1. Am J Pathol. 1982 Aug;108(2):240–245. [PMC free article] [PubMed] [Google Scholar]
- Oite T., Shimizu F., Kihara I., Batsford S. R., Vogt A. An active model of immune complex glomerulonephritis in the rat employing cationized antigen. Am J Pathol. 1983 Aug;112(2):185–194. [PMC free article] [PubMed] [Google Scholar]
- Rehan A., Johnson K. J., Kunkel R. G., Wiggins R. C. Role of oxygen radicals in phorbol myristate acetate-induced glomerular injury. Kidney Int. 1985 Mar;27(3):503–511. doi: 10.1038/ki.1985.39. [DOI] [PubMed] [Google Scholar]
- Rehan A., Johnson K. J., Wiggins R. C., Kunkel R. G., Ward P. A. Evidence for the role of oxygen radicals in acute nephrotoxic nephritis. Lab Invest. 1984 Oct;51(4):396–403. [PubMed] [Google Scholar]
- Rehan A., Wiggins R. C., Kunkel R. G., Till G. O., Johnson K. J. Glomerular injury and proteinuria in rats after intrarenal injection of cobra venom factor. Evidence for the role of neutrophil-derived oxygen free radicals. Am J Pathol. 1986 Apr;123(1):57–66. [PMC free article] [PubMed] [Google Scholar]
- Salmon J. A., Simmons P. M., Palmer R. M. A radioimmunoassay for leukotriene B4. Prostaglandins. 1982 Aug;24(2):225–235. doi: 10.1016/0090-6980(82)90148-4. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. Origin of the rat mesangial phagocyte and its expression of the leukocyte common antigen. Lab Invest. 1984 Nov;51(5):515–523. [PubMed] [Google Scholar]
- Scott W. A., Pawlowski N. A., Murray H. W., Andreach M., Zrike J., Cohn Z. A. Regulation of arachidonic acid metabolism by macrophage activation. J Exp Med. 1982 Apr 1;155(4):1148–1160. doi: 10.1084/jem.155.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg P. S., Johnson H. M., Oppenheim J. J. Regulation of murine macrophage Ia antigen expression by an immune interferon-like lymphokine: inhibitory effect of endotoxin. J Immunol. 1982 Dec;129(6):2402–2406. [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Johnson H. M., Oppenheim J. J. Regulation of murine macrophage Ia antigen expression by a lymphokine with immune interferon activity. J Exp Med. 1982 Dec 1;156(6):1780–1793. doi: 10.1084/jem.156.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura R., Werb Z. Secretory products of macrophages and their physiological functions. Am J Physiol. 1984 Jan;246(1 Pt 1):C1–C9. doi: 10.1152/ajpcell.1984.246.1.C1. [DOI] [PubMed] [Google Scholar]
- Tripp C. S., Leahy K. M., Needleman P. Thromboxane synthase is preferentially conserved in activated mouse peritoneal macrophages. J Clin Invest. 1985 Aug;76(2):898–901. doi: 10.1172/JCI112051. [DOI] [PMC free article] [PubMed] [Google Scholar]


