Abstract
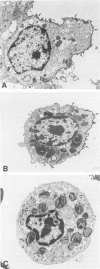
In adult rat lung the lectin Maclura pomifera agglutinin (MPA) binds apically to alveolar type II (ATII) cells but not to alveolar type I (ATI) cells. This suggests that the presence of MPA binding glycoproteins might be a criterion by which to distinguish the differentiated state of these two adult alveolar epithelial cells. The authors therefore studied MPA binding glycoproteins of ATII cells, comparing, biochemically and cytochemically, MPA binding glycoproteins in freshly isolated ATII cells with those in cultures of ATII cells that are "dedifferentiating" or have "dedifferentiated" as a result of growth on tissue culture plasticware. A MPA binding glycoprotein (185 kd) that is present in freshly isolated "differentiated" ATII cells and then is subsequently lost as isolated ATII cells "dedifferentiate" in tissue culture has been identified. This 185 kd MPA binding glycoprotein alone, or expressed in conjunction with other proteins, is a candidate for a differentiation marker for ATII cells. Preliminary data suggests that this 185 kd MPA binding glycoprotein is not found in ATI cells.
Full text
PDF


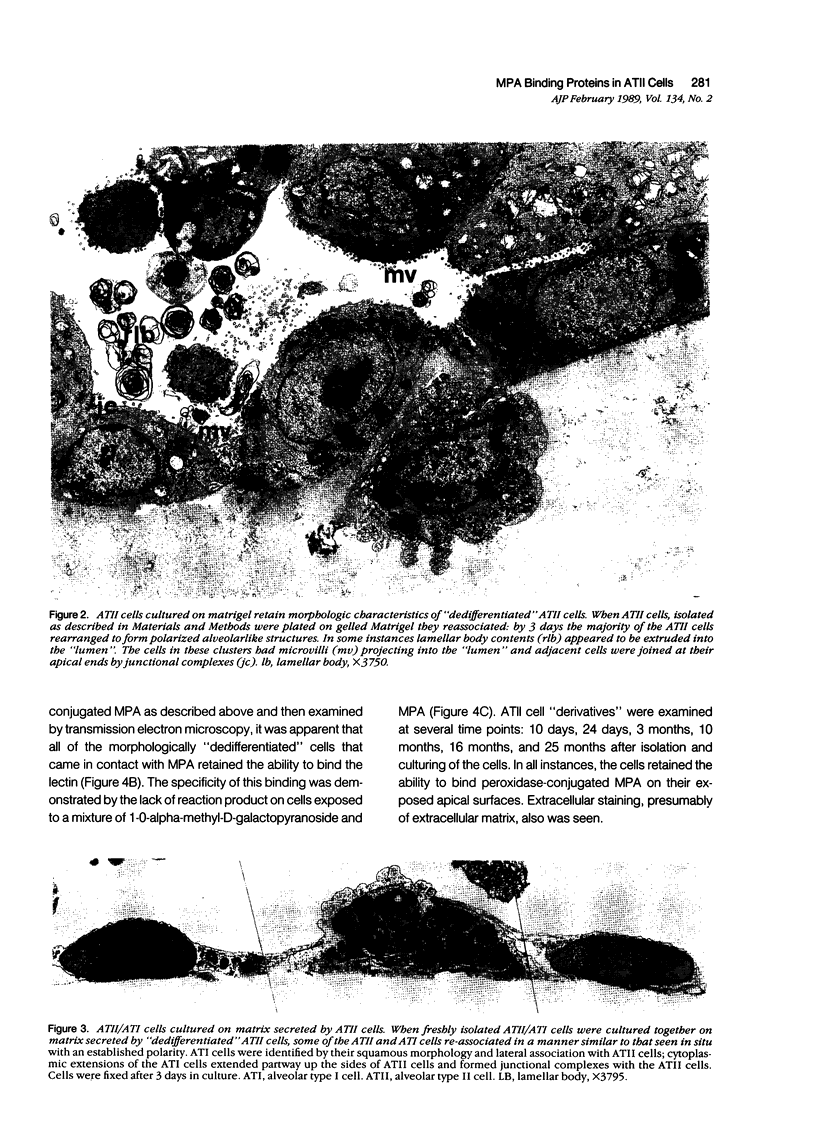

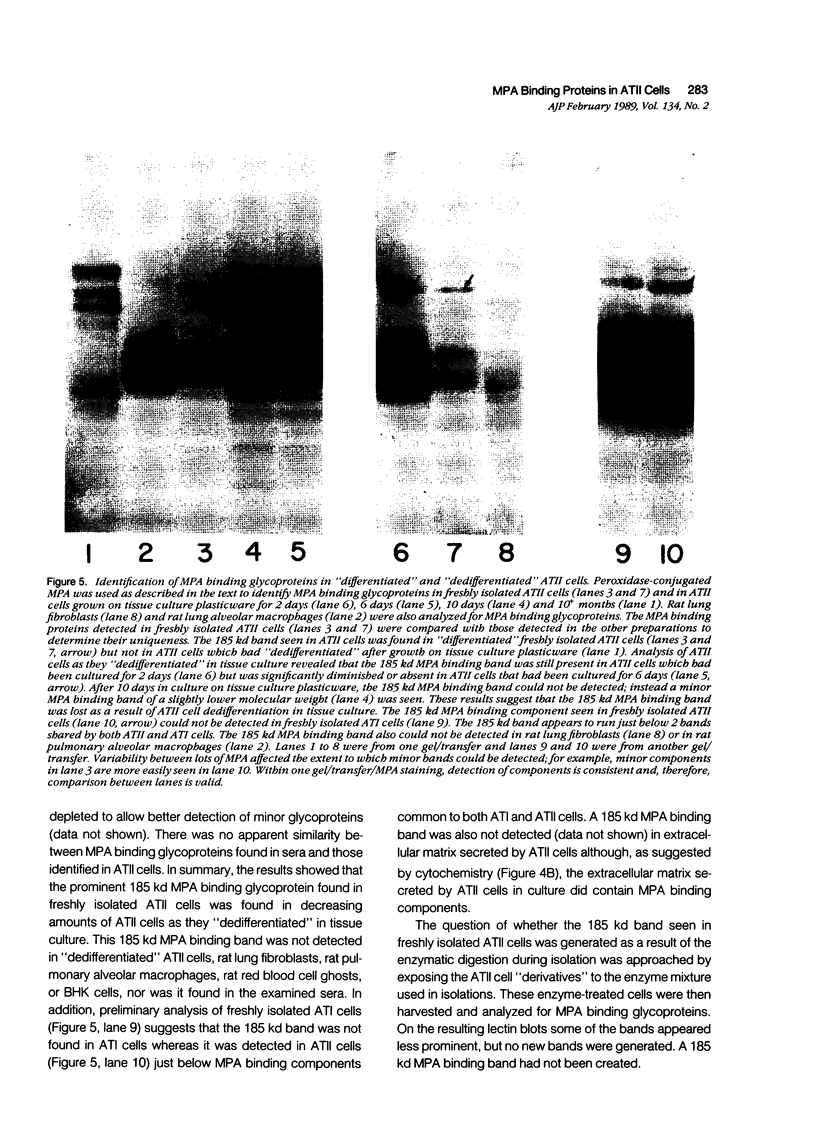


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974 Jan;30(1):35–42. [PubMed] [Google Scholar]
- Bachofen M., Weibel E. R. Basic pattern of tissue repair in human lungs following unspecific injury. Chest. 1974 Apr;65(Suppl):14S–19S. doi: 10.1378/chest.65.4_supplement.14s. [DOI] [PubMed] [Google Scholar]
- Batenburg J. J., Funkhouser J. D., Klazinga W., Van Golde L. M. On the suitability of organotypic cultures of fetal rat lung type II cells for biochemical studies concerning development. Biochim Biophys Acta. 1983 Jan 7;750(1):60–67. doi: 10.1016/0005-2760(83)90204-7. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diglio C. A., Kikkawa Y. The type II epithelial cells of the lung. IV. Adaption and behavior of isolated type II cells in culture. Lab Invest. 1977 Dec;37(6):622–631. [PubMed] [Google Scholar]
- Dobbs L. G., Williams M. C., Brandt A. E. Changes in biochemical characteristics and pattern of lectin binding of alveolar type II cells with time in culture. Biochim Biophys Acta. 1985 Jul 30;846(1):155–166. doi: 10.1016/0167-4889(85)90121-1. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Cabral L. J., Stephens R. J., Freeman G. Renewal of alveolar epithelium in the rat following exposure to NO2. Am J Pathol. 1973 Feb;70(2):175–198. [PMC free article] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Hirsch J. G., Fedorko M. E. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and "postfixation" in uranyl acetate. J Cell Biol. 1968 Sep;38(3):615–627. doi: 10.1083/jcb.38.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Martin G. R. Formation of a supramolecular complex is involved in the reconstitution of basement membrane components. Biochemistry. 1983 Oct 11;22(21):4969–4974. doi: 10.1021/bi00290a014. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meyer K. R., Witschi H., Ullrich R. L. Proliferative responses of type 2 lung epithelial cells after X rays and fission neutrons. Radiat Res. 1980 Jun;82(3):559–569. [PubMed] [Google Scholar]
- Taub M., Sato G. Growth of functional primary cultures of kidney epithelial cells in defined medium. J Cell Physiol. 1980 Nov;105(2):369–378. doi: 10.1002/jcp.1041050220. [DOI] [PubMed] [Google Scholar]
- Weller N. K., Karnovsky M. J. Improved isolation of rat lung alveolar type II cells. More representative recovery and retention of cell polarity. Am J Pathol. 1986 Jan;122(1):92–100. [PMC free article] [PubMed] [Google Scholar]
- Weller N. K., Karnovsky M. J. Isolation of pulmonary alveolar type I cells from adult rats. Am J Pathol. 1986 Sep;124(3):448–456. [PMC free article] [PubMed] [Google Scholar]