Abstract
CCAAT enhancer-binding protein-α (C/EBPα) functions as a pleiotropic transcriptional activator of adipocyte genes during adipogenesis. Nuclear factor C/EBP undifferentiated protein (CUP), an isoform of activator protein-2α (AP-2α), binds to repressive elements in the C/EBPα gene promoter, silencing the gene until late in the differentiation program. The CUP regulatory element overlaps a Sp (GT-box) element in the promoter to which Sp3 (or Sp1) can bind. Binding by Sp3 or Sp1 and CUP/AP2-α is mutually exclusive. Sp3 is a strong transcriptional activator of the C/EBPα gene promoter in 3T3-L1 preadipocytes and Schneider cells, this activation being repressed by CUP/AP-2α. Sp3 is expressed throughout differentiation, whereas CUP/AP-2α, which is expressed only by preadipocytes, is down-regulated during differentiation coincident with transcription of the C/EBPα gene. Thus, CUP/AP-2α delays access of Sp3 to the Sp regulatory element, preventing premature expression of C/EBPα and thereby interference by C/EBPα (which is antimitotic) with mitotic clonal expansion, an essential early event in the differentiation program.
Keywords: 3T3-L1 adipocyte, CEBPα undifferentiated protein, AP-2, Sp1, Sp3
During adipocyte differentiation, CCAAT enhancer-binding protein-α (C/EBPα) coordinately activates transcription of genes that give rise to the adipocyte phenotype (1–3). The C/EBPα gene itself is transcriptionally activated just before the expression of these genes (4). Studies on the transcriptional control of the C/EBPα gene during differentiation led to the identification of several cis-regulatory elements in the proximal promoter (5) and of nuclear factors that interact with these elements (6–8). Site-directed mutagenesis of the C/EBPα gene promoter revealed that one of these elements functions repressively and binds a nuclear protein, i.e., C/EBP undifferentiated protein (CUP), which is expressed by preadipocytes, but not by adipocytes (9, 10). Sequencing of tryptic peptides derived from purified CUP showed it to be an isoform of the transcription factor activator protein-2α (AP-2α) (7). Consistent with a repressive role, the kinetics of down-regulation of AP-2α protein and CUP-binding activity correspond precisely to the transcriptional activation of the C/EBPα gene (6, 7). Furthermore, cotransfection experiments with 3T3-L1 adipocytes, which express C/EBPα, established that CUP/AP-2α represses transcription of a C/EBPα promoter–reporter transgene. These findings implicated CUP/AP-2α as a repressor that functions early in the adipocyte differentiation program to prevent premature expression of the C/EBPα gene. Because C/EBPα is antimitotic, its expression must be delayed until the mitotic clonal expansion has occurred later in the differentiation program. It should be emphasized that mitotic clonal expansion is a prerequisite for adipocyte differentiation (1, 2, 11, 12).
We now report that the CUP regulatory element in the promoter of the C/EBPα gene overlaps a functional Sp (GT-box) binding site. Evidence is presented that, during the early phase of adipocyte differentiation, expression of CUP/AP-2α delays access of Sp3 (or Sp1) to the Sp binding site and, thereby, transcriptional activation of the C/EBPα gene.
Experimental Procedures
Human recombinant AP-2α and Sp1 were from Promega. Rabbit anti-human AP-2 (anti-hAP-2), anti-Sp1, and anti-Sp3 antibodies, and Sp1 [(thymidine kinase (tk)] consensus, AP-2 consensus, and AP-2 mutant oligonucleotides were from Santa Cruz Biotechnology. Cytomegalovirus (CMV)-hAP-2α (PCMX-L1-hAP-2α) was provided by R. Buettner (University of Regensberg, Regensberg, Germany), and CMV-Sp3 and PacUSp1 and PacUSp3 by G. Suske (Philipps University, Marburg, Germany). PCR-hAP-2α was prepared from plasmid pCMX-L1-hAP-2α by PCR with 5′-TGATCAGAATCCATCCGTTCACGCCGATCCATG-3′ and 5′-TGATCACTCGAGAGCAGCAGTAGCAGCAGCAG-3′ primers. PCR-amplified hAP-2α cDNA was cloned into pCR2.1 (Invitrogen). PacUhAP-2α was constructed by subcloning the hAP-2α insert (prepared by digestion of pCR-hAP-2α with BclI and XhoI) into pPacU (derived from PacUSp3 by digestion with BamHI and XhoI), an insect cell expression vector containing a Drosophila melanogaster actin 5C promoter and an ultrabithorax leader sequence at the 5′ end (13).
Cell Culture and Differentiation.
3T3-L1 preadipocytes were maintained and propagated in DMEM containing 10% (vol/vol) calf serum. Two-day postconfluent (designated day 0) cells were induced to differentiate (14) with DMEM containing 10% (vol/vol) FBS, 1 μg of insulin per ml, 1 μM dexamethasone, and 0.5 mM 3 α-isobutyl-1-methylxanthine until day 2. Cells were then fed DMEM supplemented with 10% FBS and 1 μg insulin per ml for 2 days, after which they were fed every other day with DMEM containing 10% FBS. Expression of adipocyte genes and acquisition of the adipocyte phenotype begins on day 3 and is maximal by day 8. D. melanogaster Schneider SL2 cells were grown at room temperature in Schneider's insect medium (GIBCO) supplemented with 10% heat-inactivated FCS.
Electrophoretic Mobility-Shift Analysis (EMSA).
EMSA was performed as previously described (10). For competition experiments, a 50-fold excess of unlabeled competitor oligonucleotide was added before addition of labeled oligonucleotide. DNA–protein complex dissociation experiments were initiated (after prior formation of protein-labeled oligonucleotide complexes) with a 50-fold excess of unlabeled oligonucleotide. The sequence (slash indicating the delimiting linker sequence) of the EF site oligonucleotide of the mouse C/EBPα promoter is 5′-GATC/−256CAGCGCCGCCGGGGTGGGGCTGA−234-3′ (bold indicates CUP binding site and underline the GT-box). The EF-MC binding site oligonucleotide (5′-GATC/CAGCGTTTAAAGGGTGGGGCTGA-3′) contained a mutated CUP binding site; the EF-MG binding site oligonucleotide (5′-GATC/CAGCGCCGCCGGATGCATGCTGA-3′) contained a mutated Sp GT-box binding site. The sequences of the consensus wild-type (wt) and mutant AP-2 binding site oligonucleotides were 5′-GATCGAACTGACCGCCCGCGGCCCGT-3′ and 5′-GATCGAACTGACCGCTTGCGGCCCGT-3′, respectively. The sequence of the Sp tk consensus binding site oligonucleotide was 5′-ATTCGATCGGGGCGGGGCGAGC-3′. Nuclear extracts were prepared by a modification of the protocol of NUN as described (10). Protein-complex formation was quantitated by PhosphorImager (Fuji film) in the linear response range.
Transfection and Luciferase Assays.
Fifty percent confluent 3T3-L1 preadipocytes were transiently cotransfected (calcium phosphate precipitation method; ref 15) with 0.5 μg of promoter–reporter construct containing 343 bp of 5′-flanking sequence and the entire 5′-untranslated region (UTR) of the C/EBPα gene in pGL3-BA luciferase or with the promoterless vector (10), along with a CMV-hAP-2α expression vector, a CMV-Sp3 expression vector (16), or a control vector lacking the insert. After 48 h in culture, cell extracts were prepared and assayed for luciferase activity. Drosophila SL2 cells were plated in 6-cm dishes at a density of 1 × 106 cells/dish the day before transfection. D. melanogaster SL2 cells were transiently transfected with PacUSp1, PacUSp3, PacUAP2, and PacU by using the calcium phosphate coprecipitation method as described (16, 17). Cells were scraped from culture dishes 48 h after transfection, sedimented for 5 min at 500 × g, washed in 5 ml of cold PBS, and recentrifuged. Cell pellets were resuspended in 0.5 ml of lysis buffer. After centrifugation supernatants were assayed for luciferase activity.
Results
Overlapping Sp and CUP/AP-2 Binding Sites in the C/EBPα Gene Promoter.
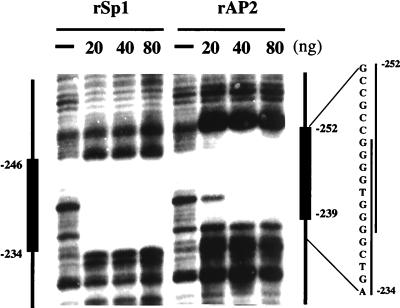
Previously (5), we observed that nuclear extract from 3T3-L1 preadipocytes footprinted the region between nucleotide −252 and nucleotide −239 in the C/EBPα promoter. The nuclear factor responsible for the footprint, referred to as CUP, was identified as the transcription factor AP-2α (7). As shown by DNase I protection (Fig. 1 Right) the region footprinted by AP-2α overlaps an adjacent GT-box (nucleotides −246 to −239). Studies by others (18, 19) have shown those members of the Sp family of transcription factors bind to certain GT-boxes. That recombinant Sp1 footprints the GT-box in the C/EBPα promoter (nucleotides −246 to −234) is verified in Fig. 1 Left. Moreover, it is evident that this footprint overlaps that by rAP-2α. These findings predict that binding by AP-2α would interfere with binding by members of the Sp family.
Figure 1.
DNase I footprinting of the C/EBPα promoter with rSp1 and rAP2. A 204-bp SmaI-StyI fragment of the C/EBPα promoter (5) was incubated with indicated amounts of rSp1 or rAP-2α and then digested with DNase I. Vertical lines on the right side indicate the footprinted sequences.
EMSA with Oligonucleotides Containing the Overlapping AP-2α and Sp Binding Sites.
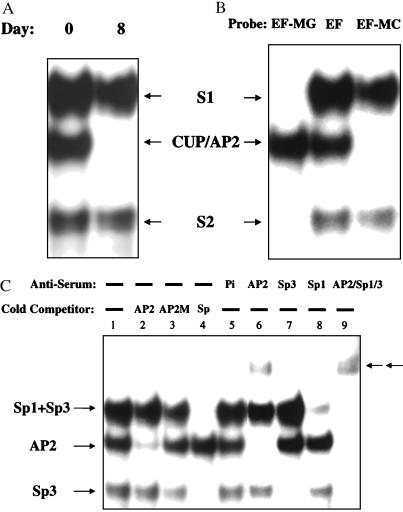
To characterize the nuclear factor(s) that binds to the GT-box adjacent to the CUP/AP-2α regulatory element in the promoter, EMSA was performed with an oligonucleotide probe, referred to as EF, that encompasses both the GT-box and the AP-2α binding site. EF was subjected to EMSA with nuclear extract from 3T3-L1 preadipocytes or adipocytes. Three protein–oligonucleotide complexes, i.e., S1, S2, and CUP/AP-2, were detected with preadipocyte nuclear extract (day 0), two of which, i.e., S1 and S2, persisted after differentiation into adipocytes (day 8) (Fig. 2A). Consistent with previous findings (6, 7), formation of the CUP/AP2-DNA complex is down-regulated during differentiation. To verify the specificity of binding, two other oligonucleotides with either a mutated CUP-binding site (EF-MC) or a mutated GT-box (EF-MG) were also subjected to EMSA with nuclear extracts from preadipocytes. Only the S1 and S2 complexes were detected with EF-MC (Fig. 2B, lane 3). The CUP/AP-2 complex was formed only with EF-MG, the oligonucleotide with a mutated GT-box (Fig. 2B, lane 1).
Figure 2.
EMSA of wt and mutant oligonucleotides containing the CUP- and/or Sp-(GT-box) binding sites with preadipocyte and adipocyte nuclear extracts. (A) 32P-end-labeled EF oligonucleotide containing the CUP- and Sp-(GT-box) binding sites of the C/EBPα gene promoter (nucleotides −258 to −234) were incubated with 10 μg of nuclear extract from 3T3-L1 preadipocytes (day 0) or adipocytes (day 8). (B) wt 32P-end-labeled EF oligonucleotide (EF), EF oligonucleotide mutated in the CUP-binding site (EF-MC), or EF oligonucleotide mutated in the GT-box were incubated with 10 μg of nuclear extract from 3T3-L1 preadipocytes. (C) Effect of wt and mutant competitor oligonucleotides and anti-AP2, -Sp1, and/or -Sp3 antibodies on EMSA with preadipocyte nuclear extract. Pi indicates preimmune serum. EMSA was performed with 32P-end-labeled EF probe. Arrows (←←) indicate the position of supershifted DNA-protein complexes.
Competition experiments were performed with unlabeled oligonucleotides corresponding to wt and mutated AP2 consensus sequences and to the tk Sp consensus sequence to which most Sp family members bind (18, 19). As above (Fig. 2 A and B), nuclear extract from preadipocytes gave rise to three protein–DNA complexes, S1, S2, and CUP/AP-2 with the EF-oligonucleotide probe (Fig.2C, lane 1). The CUP/AP-2 complex, but not S1 or S2, was competed away by an oligonucleotide corresponding to an AP-2 consensus sequence oligonucleotide (Fig. 2C, lane 2), but not by a mutated AP-2 (AP2 M) oligonucleotide (Fig. 2C, lane 3). Both S1 and S2 were competed away by the tk Sp consensus sequence oligonucleotide (Fig. 2C, lane 4).
Antibody against AP-2α completely eliminated (supershifted) the CUP/AP-2 protein–oligonucleotide complex (Fig. 2C, lane 6), whereas antibody against Sp1 removed most of the S1 protein–oligonucleotide complex, indicating that S1 is primarily due to Sp1 (Fig. 2C, lane 8). S2, which corresponds to the complex formed by the “short form” of Sp3 (20), is abolished only by antibody against Sp3 (Fig.2C, lanes 7 and 9); S1, which corresponds to the “long form” of Sp3 and to Sp1 is only partially removed by antibody against Sp3 (Fig. 2C, lane 7). Together, antibodies against Sp1, Sp3, and AP-2α completely extinguish all three complexes (Fig. 2C, lane 9).
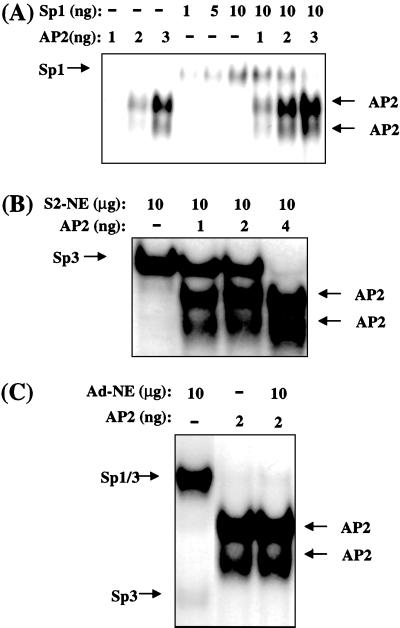
Because the Sp and AP-2 binding sites in the C/EBPα gene promoter overlap (Fig. 1), it seemed likely that binding of AP-2α and members of Sp family, notably Sp3 and Sp1, would be mutually exclusive. Because EMSA (above) was performed with “excess” oligonucleotide probe, competition between CUP/AP-2α and Sp3 or Sp1 would not have been detected. Therefore, EMSA was also performed with a “limiting” concentration of labeled EF probe. To determine whether AP-2α interferes with binding of Sp1, the effect of rAP-2α on the binding of rSp1 to the EF probe was assessed by EMSA. Increasing concentrations of rAP-2α caused decreased binding of rSp1 (Fig. 3A). Because rSp3 was unavailable, Sp3 was overexpressed in Schneider cells (SL2), which do not express Sp3, Sp1, or AP-2α (16, 21, 22), and nuclear extract from these cells was used as source of Sp3. Increasing concentrations of rAP-2α decreased the binding of Sp3 (Fig. 3B). Because fully differentiated adipocytes express both Sp1 and Sp3, but not AP-2α, adipocyte nuclear extract was also used as the source of Sp1 and Sp3. As shown in Fig. 3C, lane 3, rAP-2α competed away both Sp1 and Sp3.
Figure 3.
Effect of rAP2 on binding of rSp1 and Sp3 binding to an oligonucleotide (EF) containing overlapping CUP/AP2- and Sp-(GT-box) binding sites. EMSA was performed by using 32P-end-labeled EF oligonucleotide containing the CUP- and Sp-(GT-box) binding sites of the C/EBPα gene promoter (nucleotides −258 to −234). (A) rSp1 was incubated with increasing levels of rAP2. (B) Schneider cell nuclear extract (SL2-NE) containing Sp3 was incubated with increasing levels of rAP2. (C) 3T3-L1 adipocyte nuclear extract (Ad-NE) was incubated in the presence or absence of rAP2. The fast-moving AP-2–oligonucleotide complex is due to a proteolytic product of rAP-2 (7).
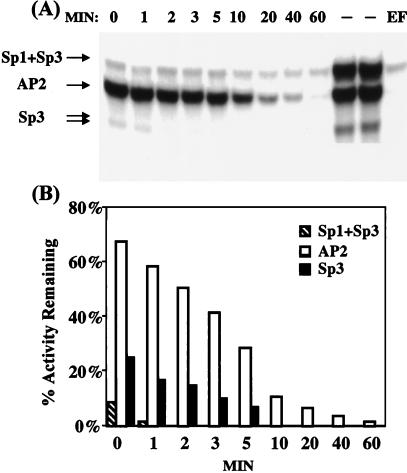
It became evident that AP-2α binds extremely tightly to the EF oligonucleotide and is difficult to compete away from the complex even with rather high levels Sp1 or Sp3 (results not shown). This observation is consistent with the relative rates of dissociation of these factors from their respective EF complexes. Comparison of these “apparent” rates by EMSA was possible because the dissociation of AP-2α from its EF complex is extremely slow. As shown in Fig. 4, the rate of dissociation of Sp3 or of Sp3 and Sp1 from the labeled EF complex is rapid relative to the displacement of CUP/AP-2α, which is extremely slow. It should be recognized that these dissociation rates represent underestimates, particularly for the Sps, because considerable dissociation of the Sps occurs during EMSA. Nevertheless, it is evident that Sp3 and Sp1 dissociate from the EF probe far more rapidly than AP-2α. The differences in these rates is consistent with much tighter binding by AP-2α than by the Sps. In view of the tightness of CUP/AP-2α binding, it is likely that Sp3 and/or Sp1 can access the GT-box and activate the C/EBPα gene only after the expression of CUP/AP-2α is down-regulated late in the differentiation program.
Figure 4.
Comparison of “apparent” rates of dissociation of AP-2α, Sp3, and Sp1 from an oligonucleotide containing CUP/AP2- and Sp-binding sites. (A) 32P-end-labeled EF oligonucleotide containing the CUP/AP2- and Sp-(GT-box) binding sites of the C/EBPα gene promoter (nucleotides −258 to −234) was incubated for 15 min at 4°C with 10 μg of 3T3-L1 preadipocyte nuclear extract and then for the indicated times, MIN (in minutes) with a 50-fold excess of unlabeled EF oligonucleotide immediately before EMSA. Gels were analyzed by phosphorimaging. Dash (–) indicates 32P-end-labeled EF without unlabeled competitor oligonucleotide; EF indicates addition of a 50-fold of unlabeled EF oligonucleotide before 32P-end-labeled EF oligonucleotide. (B) Graphic presentation of quantitative phosphorimaging results from A above.
Transactivation of the C/EBPα Promoter by Sp3 and Sp1 Is Blocked by AP-2α.
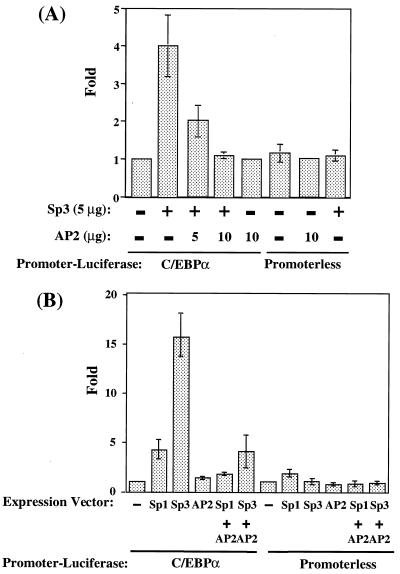
To determine whether competition between Sp3 or Sp1 and AP-2α for binding at the CUP and Sp sites can be observed in intact cells, we investigated the effect of the Sps and AP-2α on transcription mediated by the C/EBPα promoter in 3T3-L1 preadipocytes and in Drosophila Schneider cells (SL2). A 468-bp C/EBPα promoter-luciferase reporter gene (10) containing both the GT-box and CUP binding sites was cotransfected into 3T3-L1 preadipocytes and SL2 cells along with Sp3 and/or AP-2α expression vectors. In 3T3-L1 preadipocytes, Sp3 transactivated reporter gene expression about 4-fold, whereas AP-2α had no effect (Fig. 5A). However, when cotransfected with AP-2α, transactivation by Sp3 was completely abolished. Neither transcription factor had an effect on the promoterless vector.
Figure 5.
Transactivation of a C/EBPα promoter-reporter gene by Sp3 and AP-2α. Cells were cotransfected either with C/EBPα promoter-reporter or promoterless genes with or without Sp3 and/or AP-2α expression vectors. Cell lysates were prepared 48 h after transfection and assayed for luciferase activity. (A) Transfection into 3T3-L1 preadipocytes or (B) transfection into Schneider SL2
Because most vertebrate cell types, including 3T3-L1 preadipocytes, express some Sp1, Sp3, and AP-2α, detecting the effects of transfecting these transcription factors is compromised. Drosophila SL2 cells, which lack these factors (16, 21, 22), were used to circumvent this problem. As shown in Fig. 5B, transactivation (about 16-fold) by Sp3 was much higher in SL2 cells than in 3T3-L1 preadipocytes (Fig. 5A). Whereas transactivation mediated by the C/EBPα promoter occurred with both Sp3 and Sp1, transactivation by Sp3 was considerably greater than by Sp1. As was the case with 3T3-L1 cells, AP-2α dramatically repressed transactivation by either Sp3 or Sp1. These results are consistent with the binding studies described above (Fig. 3) and show that AP-2α competes effectively with Sp3 and Sp1 by inhibiting transactivation of the C/EBPα promoter in the intact cell.
Kinetics of Expression of Sp3, AP-2α, and C/EBPα During Adipocyte Differentiation.
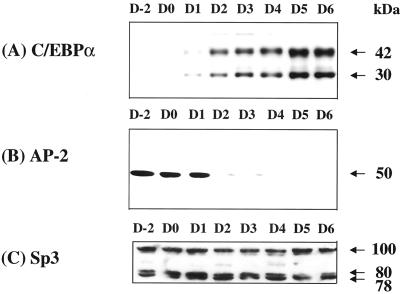
The kinetics of expression of the factors thought to be involved in the repression/derepression-activation of the C/EBPα gene during differentiation were compared. CUP/AP-2α, a repressor of the C/EBPα gene (7), is expressed before and during the mitotic clonal expansion, i.e., between days 0 and 2 (11), of the differentiation program (Fig. 6). As AP-2α begins to decline on day 2, the expression of C/EBPα is activated. Whereas the expression of Sp3 (and Sp1, not shown) does not change significantly during this critical period (days 2–6), the Sp3/AP-2α expression ratio shifts (Fig. 6). Presumably, activation by Sp3 (or Sp1) does not occur until the CUP site is vacated by AP-2α, because Sp3 or Sp1 cannot compete effectively with AP-2α. However, as [AP-2α] declines, the “apparent” Sp3/AP-2α expression ratio shifts from ≈0.5–1.0 on day 2 to >10 on day 6. This change coincides with the transcriptional activation of C/EBPα gene.
Figure 6.
Kinetics of expression of CUP/AP2, C/EBPα, and Sp3 after induction of adipocyte differentiation. 3T3-L1 preadipocytes were subjected to the differentiation protocol. Nuclear extracts were prepared at the times indicated and subjected to immunoblotting with antibody directed against either (A) C/EBPα, (B) AP-2α, or (C) Sp3. The previously published data shown in A and B were used for comparison of the kinetics of the expression of C/EBPα and AP-2α, respectively, with those of Sp3 (C). [A is modified with permission from ref. 10 (Copyright 1997, National Academy of Sciences, U.S.A.); B is modified with permission from ref. 7 (Copyright 1998, National Academy of Sciences, U.S.A.)].
Discussion
The timing of the events in the adipocyte differentiation program is critical. Mitotic clonal expansion, a required early event in the differentiation program, must precede the expression of C/EBPα, which is a pleiotropic transcriptional activator of the genes that produce the adipocyte phenotype (1, 2). The expression of C/EBPα must be delayed until after mitotic clonal expansion, because C/EBPα is antimitotic (23). Earlier work in our laboratory (7) showed that transcription of the C/EBPα gene is repressed during mitotic clonal expansion by CUP/AP-2α, which binds specifically to the proximal promoter of the gene. When mitotic clonal expansion is completed, CUP/AP-2α is down-regulated, and transcription of the C/EBPα gene is activated. Two mechanisms appear to be responsible for delaying the transcription of the C/EBPα gene until clonal expansion is complete: (i) the delayed acquisition of DNA binding activity by C/EBP-β and -δ, which are required transactivators of the C/EBPα gene (11) and, as reported here, (ii) the sequential repression and activation by CUP/AP-2α and Sp3, respectively.
The C/EBPα gene promoter possesses a functional C/EBP binding site (5) that mediates transactivation by C/EBP-β and -δ. C/EBP-β and -δ are rapidly (within a few hours) expressed after induction of differentiation. However, these transcription factors lack DNA binding activity until much later in the differentiation program (11). The inability of C/EBP-β and -δ to bind DNA appears to be due to formation of inactive heterodimers with CHOP-10 (C/EBP homologous protein 10; ref. 24), a dominant-negative inhibitor of C/EBPs (25). After a lag, CHOP-10 is down-regulated, releasing C/EBP-β and -δ from repressive constraint, which can then bind to the C/EBP regulatory element in the C/EBPα promoter and transactivate the gene.
Evidence in the present paper implicates an additional delaying action involving CUP/AP-2α and Sp family members, i.e., Sp3 and Sp1. The C/EBPα gene promoter possesses a CUP/AP-2α regulatory element that overlaps a Sp regulatory element (GT-box, Fig. 1). Whereas Sp3 (and Sp1 to a lesser extent; Fig. 5B) is a strong transactivator of the C/EBPα gene, transactivation of the gene cannot occur during mitotic clonal expansion because the Sp binding site is occluded by CUP/AP-2α. As mitotic clonal expansion ceases, however, expression of CUP/AP-2α is down-regulated, rendering the Sp/GT-box accessible to Sp3 for transcriptional activation of the gene. Thus, two repressive mechanisms ensure that the C/EBPα gene is silenced until after mitotic clonal expansion occurs, the first being the delayed acquisition of DNA binding activity by C/EBP-β and C/EBP-δ and the second repression of the gene by CUP/AP-2α.
Acknowledgments
We thank Dr. R. Buettner (University of Regensberg, Regensberg, Germany) for providing the AP-2α expression vector and Dr. G. Suske (Philipps University, Marburg, Germany) for providing the Sp3 and Sp1 expression vectors. This work was supported by a research grant from the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases).
Abbreviations
- C/EBPα
CCAAT enhancer-binding protein-α
- AP-2α
activator protein-2α
- CUP
C/EBP undifferentiated protein
- EMSA
electrophoretic mobility-shift analysis
- wt
wild-type
- tk
thymidine kinase
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.220426097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.220426097
References
- 1.Cornelius P, MacDougald O A, Lane M D. Annu Rev Nutr. 1994;14:99–129. doi: 10.1146/annurev.nu.14.070194.000531. [DOI] [PubMed] [Google Scholar]
- 2.MacDougald O A, Lane M D. Annu Rev Biochem. 1995;64:345–373. doi: 10.1146/annurev.bi.64.070195.002021. [DOI] [PubMed] [Google Scholar]
- 3.Hwang C, Loftus T, Mandrup S, Lane M. Annu Rev Cell Biol Dev Biol. 1997;13:231–259. doi: 10.1146/annurev.cellbio.13.1.231. [DOI] [PubMed] [Google Scholar]
- 4.Lane M D, Jiang M-S, Tang Q-Q. In: Pennington Nutrition Series: Nutrition, Genetics and Obesity. Bray G, Ryan D, editors. Baton Rouge, LA: Louisiana State Univ. Press; 1999. pp. 459–476. [Google Scholar]
- 5.Christy R J, Kaestner K H, Geiman D E, Lane M D. Proc Natl Acad Sci USA. 1991;88:2593–2597. doi: 10.1073/pnas.88.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasseur-Cognet M, Lane M D. Proc Natl Acad Sci USA. 1993;90:7312–7316. doi: 10.1073/pnas.90.15.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang M S, Tang Q Q, McLenithan J, Geiman D, Shillinglaw W, Henzel W J, Lane M D. Proc Natl Acad Sci USA. 1998;95:3467–3471. doi: 10.1073/pnas.95.7.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Q-Q, Jiang M-S, Lane M D. Mol Cell Biol. 1999;19:4855–4865. doi: 10.1128/mcb.19.7.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasseur-Cognet M, Lane M D. Curr Opin Genet Dev. 1993;3:238–245. doi: 10.1016/0959-437x(93)90029-o. [DOI] [PubMed] [Google Scholar]
- 10.Tang Q Q, Jiang M S, Lane M D. Proc Natl Acad Sci USA. 1997;94:13571–13575. doi: 10.1073/pnas.94.25.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Q-Q, Lane M D. Genes Dev. 1999;13:2231–2241. doi: 10.1101/gad.13.17.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel Y M, Lane M D. J Biol Chem. 2000;275:17653–17660. doi: 10.1074/jbc.M910445199. [DOI] [PubMed] [Google Scholar]
- 13.Dennig J, Beato M, Suske G. EMBO J. 1996;15:5659–5667. [PMC free article] [PubMed] [Google Scholar]
- 14.Student A K, Hsu R Y, Lane M D. J Biol Chem. 1980;255:4745–4750. [PubMed] [Google Scholar]
- 15.Hwang C-S, Mandrup S, MacDougald O M, Geiman D E, Lane M D. Proc Natl Acad Sci USA. 1996;93:873–877. doi: 10.1073/pnas.93.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagen G, Muller S, Beato M, Suske G. EMBO J. 1994;13:3843–3851. doi: 10.1002/j.1460-2075.1994.tb06695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suske G. Methods Mol Biol. 2000;130:175–187. doi: 10.1385/1-59259-686-x:175. [DOI] [PubMed] [Google Scholar]
- 18.Hagen G, Muller S, Beato M, Suske G. Nucleic Acids Res. 1992;20:5519–5525. doi: 10.1093/nar/20.21.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kingsley C, Winoto A. Mol Cell Biol. 1992;12:4251–4261. doi: 10.1128/mcb.12.10.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennett S B, Udvadia A J, Horowitz J M. Nucleic Acids Res. 1997;25:3110–3117. doi: 10.1093/nar/25.15.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagen G, Dennig J, Preiss A, Beato M, Suske G. J Biol Chem. 1995;270:24989–24994. doi: 10.1074/jbc.270.42.24989. [DOI] [PubMed] [Google Scholar]
- 22.Birnbaum M J, van Wijnen A J, Odgren P R, Last T J, Suske G, Stein G S, Stein J L. Biochemistry. 1995;34:16503–16508. doi: 10.1021/bi00050a034. [DOI] [PubMed] [Google Scholar]
- 23.Umek R M, Friedman A D, McKnight S L. Science. 1991;251:288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- 24.Tang, Q. Q. & Lane, M. D. (2000) Proc. Natl. Acad. Sci. USA 97, in press.
- 25.Ron D, Habener J F. Genes Dev. 1992;6:439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]