Abstract
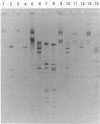
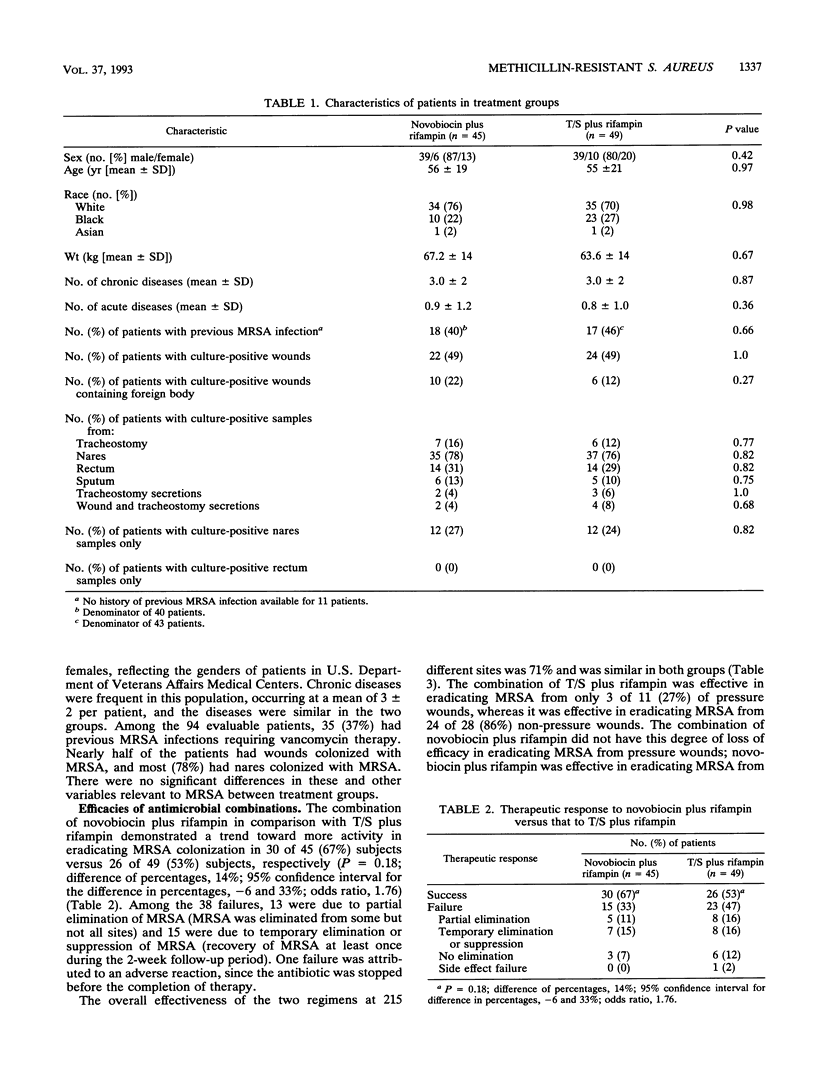
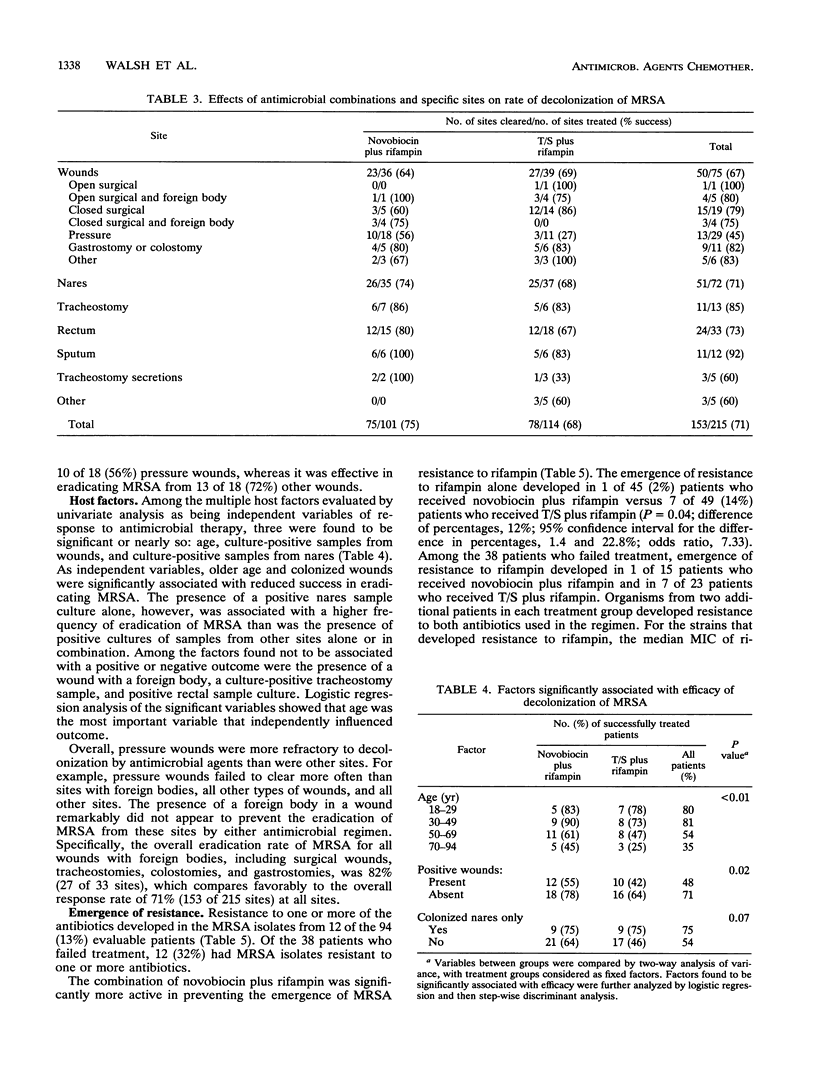
Methicillin-resistant Staphylococcus aureus (MRSA) is a major pathogen in hospitals. Current antimicrobial regimens for eradicating colonizing strains are not well defined and are often complicated by the emergence of resistance. The combination of novobiocin plus rifampin in vitro and in vivo was found to prevent the emergence of resistant populations of initially susceptible strains of MRSA, particularly resistance to rifampin. We therefore studied, in a randomized, double-blind, multicenter comparative trial, the combination of novobiocin plus rifampin versus trimethoprim-sulfamethoxazole (T/S) plus rifampin in order to determine the efficacy of each regimen in eradicating MRSA colonization and to further characterize the host factors involved in the response to this antimicrobial therapy. Among the 126 individuals enrolled in the study, 94 (80 patients; 14 hospital personnel) were evaluable. Among the 94 evaluable subjects, no significant demographic or medical differences existed between the two treatment groups. Successful clearance of the colonizing MRSA strains was achieved in 30 of 45 (67%) subjects receiving novobiocin plus rifampin, whereas successful clearance was achieved in 26 of 49 (53%) subjects treated with T/S plus rifampin (P = 0.18). The emergence of resistance to rifampin developed more frequently in 14% (7 of 49) of subjects treated with T/S plus rifampin than in 2% (1 of 45) of subjects treated with novobiocin plus rifampin (P = 0.04). Restriction endonuclease studies of large plasmid DNA demonstrated that the same strain was present at pretherapy and posttherapy in most refractory cases (24 of 29 [83%] subjects). Among the 56 successfully treated subjects, clearance of MRSA was age dependent: 29 of 36 (80%) subjects in the 18- to 49-year-old age group, 19 of 35 (54%) subjects in the 50- to 69-year-old age group, and 8 of 23 (35%) in the 70- to 94-year-old age group (P < 0.01). Clearance was also site dependent; culture-positive samples from wounds were related to a successful outcome in only 22 (48%) of 46 subjects, whereas culture-positive samples from sites other than wounds (e.g., nares, rectum, and sputum) were associated with a success rate of 34 of 48 (71%) subjects (P = 0.02). Foreign bodies in wounds did not prevent the eradication of MRSA by either regimen. T/S plus rifampin was less effective in clearing both pressure and other wounds, whereas novobiocin plus rifampin was equally effective in clearing both pressure and other wounds. There were no significant differences in toxicity between the two regimens. Thus, the combination of novobiocin plus rifampin, in comparison with T/S plus rifampin, was more effective in preventing the emergence of resistance to rifampin and demonstrated a trend toward greater activity in clearing the MRSA carrier state. The response to either combination depended on host factors, particularly age and the site of MRSA colonization.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arathoon E. G., Hamilton J. R., Hench C. E., Stevens D. A. Efficacy of short courses of oral novobiocin-rifampin in eradicating carrier state of methicillin-resistant Staphylococcus aureus and in vitro killing studies of clinical isolates. Antimicrob Agents Chemother. 1990 Sep;34(9):1655–1659. doi: 10.1128/aac.34.9.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIDGES R. A., BERENDES H., GOOD R. A. Serious reactions to novobiocin. J Pediatr. 1957 May;50(5):579–585. doi: 10.1016/s0022-3476(57)80222-4. [DOI] [PubMed] [Google Scholar]
- Bacon A. E., Jorgensen K. A., Wilson K. H., Kauffman C. A. Emergence of nosocomial methicillin-resistant Staphylococcus aureus and therapy of colonized personnel during a hospital-wide outbreak. Infect Control. 1987 Apr;8(4):145–150. doi: 10.1017/s0195941700065802. [DOI] [PubMed] [Google Scholar]
- Boyce J. M., Causey W. A. Increasing occurrence of methicillin-resistant Staphylococcus aureus in the United States. Infect Control. 1982 Sep-Oct;3(5):377–383. doi: 10.1017/s0195941700057337. [DOI] [PubMed] [Google Scholar]
- Canawati H. N., Tuddenham W. J., Sapico F. L., Montgomerie J. Z., Aeilts G. D. Failure of rifampin to eradicate methicillin-resistant Staphylococcus aureus colonization. Clin Ther. 1982;4(6):526–531. [PubMed] [Google Scholar]
- Cederna J. E., Terpenning M. S., Ensberg M., Bradley S. F., Kauffman C. A. Staphylococcus aureus nasal colonization in a nursing home: eradication with mupirocin. Infect Control Hosp Epidemiol. 1990 Jan;11(1):13–16. doi: 10.1086/646072. [DOI] [PubMed] [Google Scholar]
- Chow J. W., Yu V. L. Staphylococcus aureus nasal carriage in hemodialysis patients. Its role in infection and approaches to prophylaxis. Arch Intern Med. 1989 Jun;149(6):1258–1262. [PubMed] [Google Scholar]
- Craven D. E., Reed C., Kollisch N., DeMaria A., Lichtenberg D., Shen K., McCabe W. R. A large outbreak of infections caused by a strain of Staphylococcus aureus resistant of oxacillin and aminoglycosides. Am J Med. 1981 Jul;71(1):53–58. doi: 10.1016/0002-9343(81)90258-8. [DOI] [PubMed] [Google Scholar]
- Craven D. E., Rixinger A. I., Goularte T. A., McCabe W. R. Methicillin-resistant Staphylococcus aureus bacteremia linked to intravenous drug abusers using a "shooting gallery". Am J Med. 1986 May;80(5):770–776. doi: 10.1016/0002-9343(86)90614-5. [DOI] [PubMed] [Google Scholar]
- Drusano G. L., Townsend R. J., Walsh T. J., Forrest A., Antal E. J., Standiford H. C. Steady-state serum pharmacokinetics of novobiocin and rifampin alone and in combination. Antimicrob Agents Chemother. 1986 Jul;30(1):42–45. doi: 10.1128/aac.30.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison R. T., 3rd, Judson F. N., Peterson L. C., Cohn D. L., Ehret J. M. Oral rifampin and trimethoprim/sulfamethoxazole therapy in asymptomatic carriers of methicillin-resistant Staphylococcus aureus infections. West J Med. 1984 May;140(5):735–740. [PMC free article] [PubMed] [Google Scholar]
- Garner J. S., Simmons B. P. Guideline for isolation precautions in hospitals. Infect Control. 1983 Jul-Aug;4(4 Suppl):245–325. [PubMed] [Google Scholar]
- Hackbarth C. J., Chambers H. F. Methicillin-resistant staphylococci: detection methods and treatment of infections. Antimicrob Agents Chemother. 1989 Jul;33(7):995–999. doi: 10.1128/aac.33.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley R. W., Hightower A. W., Khabbaz R. F., Thornsberry C., Martone W. J., Allen J. R., Hughes J. M. The emergence of methicillin-resistant Staphylococcus aureus infections in United States hospitals. Possible role of the house staff-patient transfer circuit. Ann Intern Med. 1982 Sep;97(3):297–308. doi: 10.7326/0003-4819-97-3-297. [DOI] [PubMed] [Google Scholar]
- Hansen S. L., Walsh T. J. Detection of intrinsically resistant (heteroresistant) Staphylococcus aureus with the Sceptor and AutoMicrobic systems. J Clin Microbiol. 1987 Feb;25(2):412–415. doi: 10.1128/jcm.25.2.412-415.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. L., Duckworth G. J., Casewell M. W. Elimination of nasal carriage of methicillin-resistant Staphylococcus aureus with mupirocin during a hospital outbreak. J Antimicrob Chemother. 1988 Sep;22(3):377–384. doi: 10.1093/jac/22.3.377. [DOI] [PubMed] [Google Scholar]
- Johnston B. L., Kwok R. Y., Mulligan M. E. In vitro activity of novobiocin and rifampin alone and in combination against oxacillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. 1987 Nov;8(3):137–147. doi: 10.1016/0732-8893(87)90164-7. [DOI] [PubMed] [Google Scholar]
- KIRBY W. M., HUDSON D. G., NOYES W. D. Clinical and laboratory studies of novobiocin, a new antibiotic. AMA Arch Intern Med. 1956 Jul;98(1):1–7. doi: 10.1001/archinte.1956.00250250007001. [DOI] [PubMed] [Google Scholar]
- LUBASH G., VAN DER MEULEN J., BERNTSEN C., Jr, TOMPSETT R. Novobiocin: a laboratory investigation. Antibiotic Med Clin Ther (New York) 1956 Apr;2(4):233–240. [PubMed] [Google Scholar]
- Milatovic D. Vancomycin for treatment of infections with methicillin-resistant Staphylococcus aureus: are there alternatives? Eur J Clin Microbiol. 1986 Dec;5(6):689–692. doi: 10.1007/BF02013306. [DOI] [PubMed] [Google Scholar]
- Muder R. R., Brennen C., Wagener M. M., Vickers R. M., Rihs J. D., Hancock G. A., Yee Y. C., Miller J. M., Yu V. L. Methicillin-resistant staphylococcal colonization and infection in a long-term care facility. Ann Intern Med. 1991 Jan 15;114(2):107–112. doi: 10.7326/0003-4819-114-2-1-107. [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Ruane P. J., Johnston L., Wong P., Wheelock J. P., MacDonald K., Reinhardt J. F., Johnson C. C., Statner B., Blomquist I. Ciprofloxacin for eradication of methicillin-resistant Staphylococcus aureus colonization. Am J Med. 1987 Apr 27;82(4A):215–219. [PubMed] [Google Scholar]
- Myers J. P., Linnemann C. C., Jr Bacteremia due to methicillin-resistant Staphylococcus aureus. J Infect Dis. 1982 Apr;145(4):532–536. doi: 10.1093/infdis/145.4.532. [DOI] [PubMed] [Google Scholar]
- NICHOLS R. L., FINLAND M. Novobiocin; a limited bacteriologic and clinical study of its use in forty-five patients. Antibiotic Med Clin Ther (New York) 1956 Apr;2(4):241–257. [PubMed] [Google Scholar]
- Newman R., Doster B. E., Murray F. J., Woolpert S. F. Rifampin in initial treatment of pulmonary tuberculosis. A.U.S. Public Health Service tuberculosis therapy trial. Am Rev Respir Dis. 1974 Feb;109(2):216–232. doi: 10.1164/arrd.1974.109.2.216. [DOI] [PubMed] [Google Scholar]
- PULASKI E. J., ISOKANE R. K. Novobiocin therapy of pyogenic surgical infections. Surg Gynecol Obstet. 1957 Mar;104(3):310–318. [PubMed] [Google Scholar]
- Peacock J. E., Jr, Moorman D. R., Wenzel R. P., Mandell G. L. Methicillin-resistant Staphylococcus aureus: microbiologic characteristics, antimicrobial susceptibilities, and assessment of virulence of an epidemic strain. J Infect Dis. 1981 Dec;144(6):575–582. doi: 10.1093/infdis/144.6.575. [DOI] [PubMed] [Google Scholar]
- Reagan D. R., Doebbeling B. N., Pfaller M. A., Sheetz C. T., Houston A. K., Hollis R. J., Wenzel R. P. Elimination of coincident Staphylococcus aureus nasal and hand carriage with intranasal application of mupirocin calcium ointment. Ann Intern Med. 1991 Jan 15;114(2):101–106. doi: 10.7326/0003-4819-114-2-101. [DOI] [PubMed] [Google Scholar]
- Rhinehart E., Shlaes D. M., Keys T. F., Serkey J., Kirkley B., Kim C., Currie-McCumber C. A., Hall G. Nosocomial clonal dissemination of methicillin-resistant Staphylococcus aureus. Elucidation by plasmid analysis. Arch Intern Med. 1987 Mar;147(3):521–524. [PubMed] [Google Scholar]
- Ribner B. S., Landry M. N., Gholson G. L. Strict versus modified isolation for prevention of nosocomial transmission of methicillin-resistant Staphylococcus aureus. Infect Control. 1986 Jun;7(6):317–320. doi: 10.1017/s0195941700064341. [DOI] [PubMed] [Google Scholar]
- Rimland D., Roberson B. Gastrointestinal carriage of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1986 Jul;24(1):137–138. doi: 10.1128/jcm.24.1.137-138.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccaforte J. S., Bittner M. J., Stumpf C. A., Preheim L. C. Attempts to eradicate methicillin-resistant Staphylococcus aureus colonization with the use of trimethoprim-sulfamethoxazole, rifampin, and bacitracin. Am J Infect Control. 1988 Aug;16(4):141–146. doi: 10.1016/0196-6553(88)90024-7. [DOI] [PubMed] [Google Scholar]
- Rode H., Hanslo D., de Wet P. M., Millar A. J., Cywes S. Efficacy of mupirocin in methicillin-resistant Staphylococcus aureus burn wound infection. Antimicrob Agents Chemother. 1989 Aug;33(8):1358–1361. doi: 10.1128/aac.33.8.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sande M. A., Mandell G. L. Effect of rifampin on nasal carriage of Staphylococcus aureus. Antimicrob Agents Chemother. 1975 Mar;7(3):294–297. doi: 10.1128/aac.7.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standiford H. C. Methicillin-resistant Staphylococcus aureus infections: it's time to get tough. Infect Control. 1987 May;8(5):187–189. doi: 10.1017/s0195941700065917. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Wenzel R. P. International recognition of methicillin-resistant strains of Staphylococcus aureus. Ann Intern Med. 1982 Dec;97(6):925–926. doi: 10.7326/0003-4819-97-6-925. [DOI] [PubMed] [Google Scholar]
- WELCH H., LEWIS C. N., PUTNAM L. E., RANDALL W. A. A study of the sensitizing potential of novobiocin. Antibiotic Med Clin Ther (New York) 1956 Jun;3(1):27–32. [PubMed] [Google Scholar]
- Walsh T. J., Auger F., Tatem B. A., Hansen S. L., Standiford H. C. Novobiocin and rifampicin in combination against methicillin-resistant Staphylococcus aureus: an in-vitro comparison with vancomycin plus rifampicin. J Antimicrob Chemother. 1986 Jan;17(1):75–82. doi: 10.1093/jac/17.1.75. [DOI] [PubMed] [Google Scholar]
- Walsh T. J., Hansen S. L., Tatem B. A., Auger F., Standiford H. C. Activity of novobiocin against methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1985 Apr;15(4):435–440. doi: 10.1093/jac/15.4.435. [DOI] [PubMed] [Google Scholar]
- Walsh T. J., Vlahov D., Hansen S. L., Sonnenberg E., Khabbaz R., Gadacz T., Standiford H. C. Prospective microbiologic surveillance in control of nosocomial methicillin-resistant Staphylococcus aureus. Infect Control. 1987 Jan;8(1):7–14. doi: 10.1017/s0195941700066923. [DOI] [PubMed] [Google Scholar]
- Ward T. T., Winn R. E., Hartstein A. I., Sewell D. L. Observations relating to an inter-hospital outbreak of methicillin-resistant Staphylococcus aureus: role of antimicrobial therapy in infection control. Infect Control. 1981 Nov-Dec;2(6):453–459. doi: 10.1017/s0195941700055715. [DOI] [PubMed] [Google Scholar]
- Watanakunakorn C. Treatment of infections due to methicillin-resistant Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):376–378. doi: 10.7326/0003-4819-97-3-376. [DOI] [PubMed] [Google Scholar]
- Wenzel R. P. Methicillin-resistant S. aureus and S. epidermidis strains: modern hospital pathogens. Infect Control. 1986 Feb;7(2 Suppl):118–119. doi: 10.1017/s0195941700065620. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Tamura Y., Yokota T. Antiseptic and antibiotic resistance plasmid in Staphylococcus aureus that possesses ability to confer chlorhexidine and acrinol resistance. Antimicrob Agents Chemother. 1988 Jun;32(6):932–935. doi: 10.1128/aac.32.6.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V. L., Goetz A., Wagener M., Smith P. B., Rihs J. D., Hanchett J., Zuravleff J. J. Staphylococcus aureus nasal carriage and infection in patients on hemodialysis. Efficacy of antibiotic prophylaxis. N Engl J Med. 1986 Jul 10;315(2):91–96. doi: 10.1056/NEJM198607103150204. [DOI] [PubMed] [Google Scholar]