Abstract
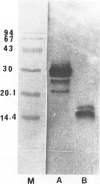
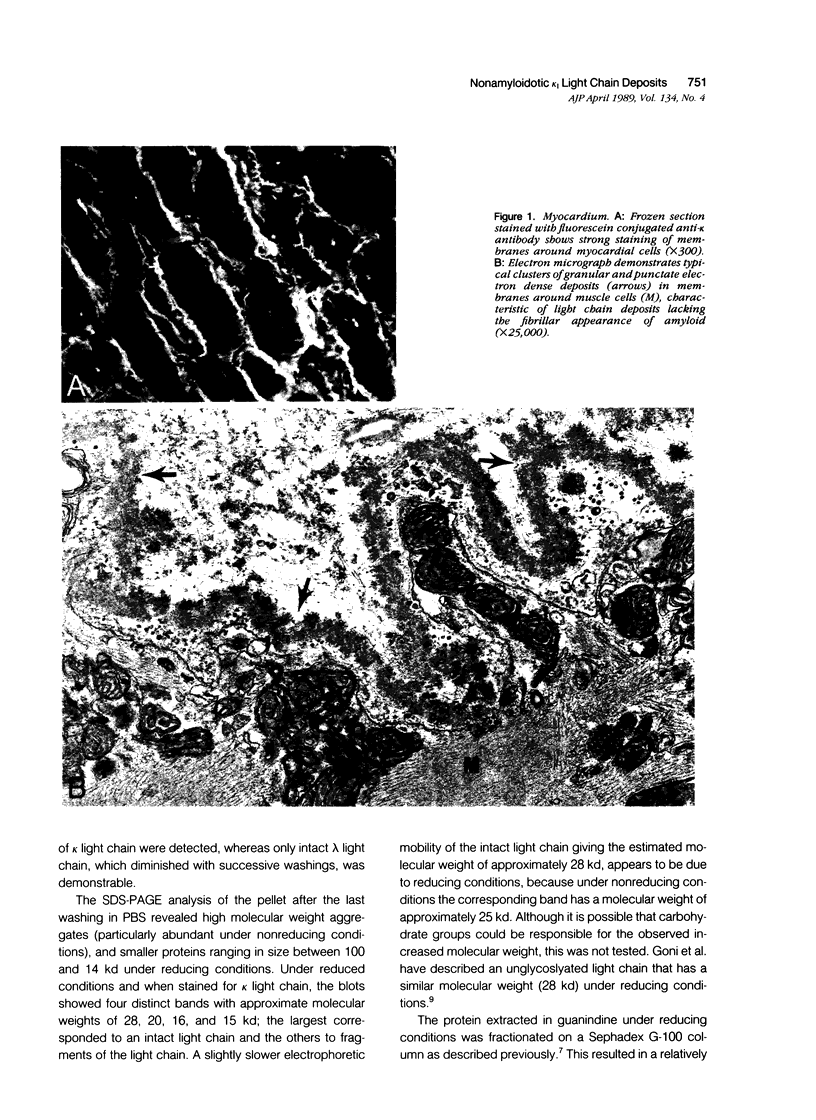
The authors biochemically analyzed the nonamyloidotic light chain deposits, the first studied in this way, from a patient with systemic kappa light chain deposition disease (LCDD). The light chain deposits from myocardium were extracted in 6 M guanidine-HCl under reducing conditions, partially purified by column chromatography, and analyzed by immunoblotting and amino-terminal sequencing. The extracted material contained four main bands reactive with anti-kappa antibody: intact kappa light chain (MW, 28 kd), under reducing conditions, and 3 fragments (MW, 20, 16, and 15 kd). As revealed by the aminoterminal sequencing performed on three of the four bands, the intact light chain molecule and two fragments belong to the kappa I subgroup. Thus, similar to light chain amyloid (AL), the deposits in LCDD are derived from both intact light chain and fragments. Unlike in AL, amyloid P component was not detected in the deposits of this patient or those examined previously. The differences demonstrated thus far between AL and LCDD are the lack of fibrils and amyloid P component in LCDD, suggesting that local tissue factors may be responsible for different processing of the light chain deposits in LCDD.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrad M. A., Kisilevsky R., Willmer J., Chen S. J., Skinner M. Further characterization of amyloid-enhancing factor. Lab Invest. 1982 Aug;47(2):139–146. [PubMed] [Google Scholar]
- Dwulet F. E., O'Connor T. P., Benson M. D. Polymorphism in a kappa I primary (AL) amyloid protein (BAN). Mol Immunol. 1986 Jan;23(1):73–78. doi: 10.1016/0161-5890(86)90173-2. [DOI] [PubMed] [Google Scholar]
- Gallo G. R., Caulin-Glaser T., Lamm M. E. Charge of circulating immune complexes as a factor in glomerular basement membrane localization in mice. J Clin Invest. 1981 May;67(5):1305–1313. doi: 10.1172/JCI110159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G. R., Feiner H. D., Buxbaum J. N. The kidney in lymphoplasmacytic disorders. Pathol Annu. 1982;17(Pt 1):291–317. [PubMed] [Google Scholar]
- Gallo G., Picken M., Frangione B., Buxbaum J. Nonamyloidotic monoclonal immunoglobulin deposits lack amyloid P component. Mod Pathol. 1988 Nov;1(6):453–456. [PubMed] [Google Scholar]
- Glenner G. G., Terry W., Harada M., Isersky C., Page D. Amyloid fibril proteins: proof of homology with immunoglobulin light chains by sequence analyses. Science. 1971 Jun 11;172(3988):1150–1151. doi: 10.1126/science.172.3988.1150. [DOI] [PubMed] [Google Scholar]
- Goñi F., Chuba J., Buxbaum J., Frangione B. A double monoclonal IgG1 kappa and IgG2 kappa in a single myeloma patient. Variation in clonal products and therapeutic responses. J Immunol. 1988 Jan 15;140(2):551–557. [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1303–1307. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J. B., Skinner M., Benson M. D., Cohen A. S. Fractionation of primary amyloid fibrils. Characterization and chemical interaction of the subunits. Biochim Biophys Acta. 1977 Mar 28;491(1):167–176. doi: 10.1016/0005-2795(77)90053-8. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Palant C. E., Bonitati J., Bartholomew W. R., Brentjens J. R., Walshe J. J., Bentzel C. J. Nodular glomerulosclerosis associated with multiple myeloma. Role of light chain isoelectric point. Am J Med. 1986 Jan;80(1):98–102. doi: 10.1016/0002-9343(86)90055-0. [DOI] [PubMed] [Google Scholar]
- Picken M. M., Gallo G., Buxbaum J., Frangione B. Characterization of renal amyloid derived from the variable region of the lambda light chain subgroup II. Am J Pathol. 1986 Jul;124(1):82–87. [PMC free article] [PubMed] [Google Scholar]
- Picken M. M., Pelton K., Frangione B., Gallo G. Primary amyloidosis A. Immunohistochemical and biochemical characterization. Am J Pathol. 1987 Dec;129(3):536–542. [PMC free article] [PubMed] [Google Scholar]
- Prelli F., Castaño E., Glenner G. G., Frangione B. Differences between vascular and plaque core amyloid in Alzheimer's disease. J Neurochem. 1988 Aug;51(2):648–651. doi: 10.1111/j.1471-4159.1988.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Randall R. E., Williamson W. C., Jr, Mullinax F., Tung M. Y., Still W. J. Manifestations of systemic light chain deposition. Am J Med. 1976 Feb;60(2):293–299. doi: 10.1016/0002-9343(76)90440-x. [DOI] [PubMed] [Google Scholar]
- Toyoda M., Kajita A., Kita S., Osamura Y., Shinoda T. An autopsy case of diffuse myelomatosis associated with systemic kappa light chain deposition disease (LCDD). A patho-anatomical, immunohistochemical and immunobiochemical study. Acta Pathol Jpn. 1988 Apr;38(4):479–488. doi: 10.1111/j.1440-1827.1988.tb02321.x. [DOI] [PubMed] [Google Scholar]
- Westermark P., Sletten K., Natvig J. B. Structure and antigenic behaviour of kappa I-immunoglobulin light-chain amyloid proteins. Acta Pathol Microbiol Scand C. 1981 Jun;89(3):199–203. doi: 10.1111/j.1699-0463.1981.tb02686.x. [DOI] [PubMed] [Google Scholar]