Abstract
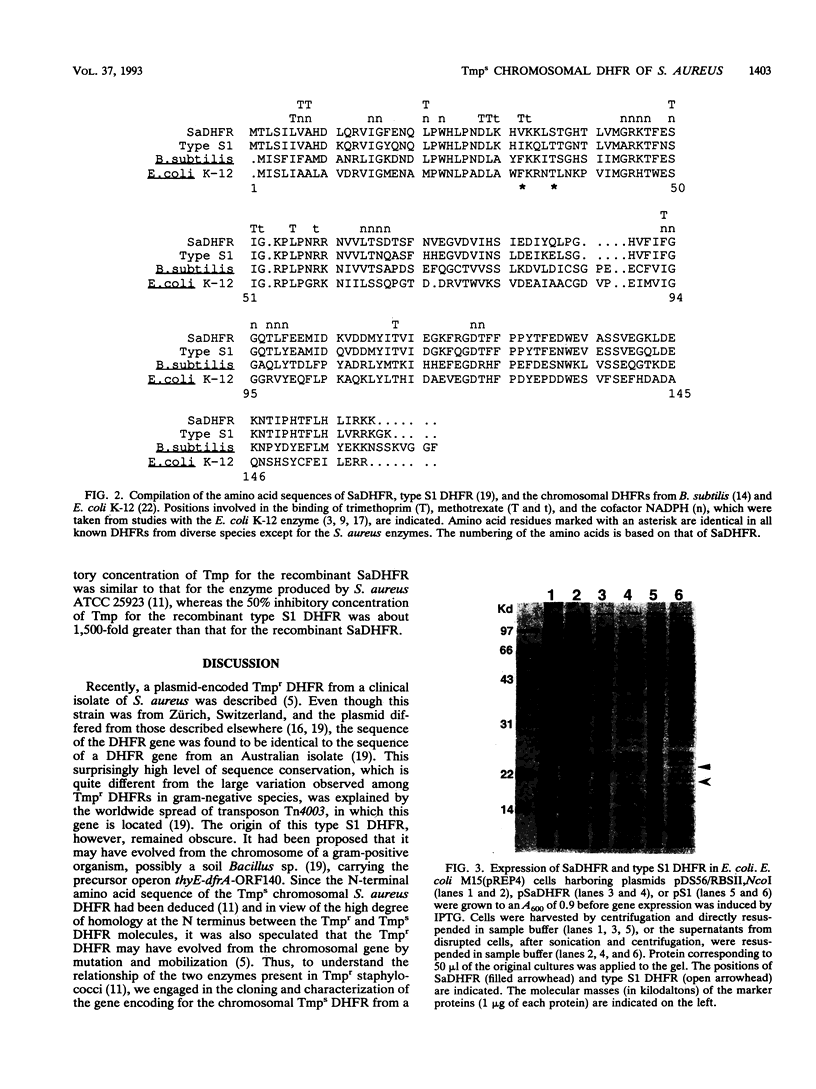
The gene for the trimethoprim-sensitive (Tmps) chromosomal dihydrofolate reductase (DHFR) of Staphylococcus aureus ATCC 25923 was cloned and characterized. The structural gene encodes a polypeptide of 159 amino acid residues and has a calculated molecular weight of 18,251. The amino acid sequences of this Tmps DHFR and those of the trimethoprim-resistant type S1 DHFR encoded by transposon Tn4003 are 80% identical. In contrast to the trimethoprim-resistant enzyme, the Tmps DHFR can be highly overexpressed in Escherichia coli, with most of the recombinant protein occurring in a soluble and an active form.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baccanari D. P., Joyner S. S. Dihydrofolate reductase hysteresis and its effect of inhibitor binding analyses. Biochemistry. 1981 Mar 31;20(7):1710–1716. doi: 10.1021/bi00510a002. [DOI] [PubMed] [Google Scholar]
- Bennett P. M., Heritage J., Hawkey P. M. An ultra-rapid method for the study of antibiotic resistance plasmids. J Antimicrob Chemother. 1986 Sep;18(3):421–424. doi: 10.1093/jac/18.3.421. [DOI] [PubMed] [Google Scholar]
- Bolin J. T., Filman D. J., Matthews D. A., Hamlin R. C., Kraut J. Crystal structures of Escherichia coli and Lactobacillus casei dihydrofolate reductase refined at 1.7 A resolution. I. General features and binding of methotrexate. J Biol Chem. 1982 Nov 25;257(22):13650–13662. [PubMed] [Google Scholar]
- Burchall J. J., Hitchings G. H. Inhibitor binding analysis of dihydrofolate reductases from various species. Mol Pharmacol. 1965 Sep;1(2):126–136. [PubMed] [Google Scholar]
- Burdeska A., Ott M., Bannwarth W., Then R. L. Identical genes for trimethoprim-resistant dihydrofolate reductase from Staphylococcus aureus in Australia and central Europe. FEBS Lett. 1990 Jun 18;266(1-2):159–162. doi: 10.1016/0014-5793(90)81529-w. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filman D. J., Bolin J. T., Matthews D. A., Kraut J. Crystal structures of Escherichia coli and Lactobacillus casei dihydrofolate reductase refined at 1.7 A resolution. II. Environment of bound NADPH and implications for catalysis. J Biol Chem. 1982 Nov 25;257(22):13663–13672. [PubMed] [Google Scholar]
- Foster T. J. Plasmid-determined resistance to antimicrobial drugs and toxic metal ions in bacteria. Microbiol Rev. 1983 Sep;47(3):361–409. doi: 10.1128/mr.47.3.361-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman P. G., Stähli M., Kocher H. P., Then R. L. N-terminal amino acid sequence of the chromosomal dihydrofolate reductase purified from trimethoprim-resistant Staphylococcus aureus. FEBS Lett. 1988 Dec 19;242(1):157–160. doi: 10.1016/0014-5793(88)81006-8. [DOI] [PubMed] [Google Scholar]
- Henner D. J., Yang M., Ferrari E. Localization of Bacillus subtilis sacU(Hy) mutations to two linked genes with similarities to the conserved procaryotic family of two-component signalling systems. J Bacteriol. 1988 Nov;170(11):5102–5109. doi: 10.1128/jb.170.11.5102-5109.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura M., Kawata M., Tsuda K., Tanaka T. Nucleotide sequence of the thymidylate synthase B and dihydrofolate reductase genes contained in one Bacillus subtilis operon. Gene. 1988 Apr 15;64(1):9–20. doi: 10.1016/0378-1119(88)90476-3. [DOI] [PubMed] [Google Scholar]
- Lee C. C., Wu X. W., Gibbs R. A., Cook R. G., Muzny D. M., Caskey C. T. Generation of cDNA probes directed by amino acid sequence: cloning of urate oxidase. Science. 1988 Mar 11;239(4845):1288–1291. doi: 10.1126/science.3344434. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., Skurray R. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev. 1987 Mar;51(1):88–134. doi: 10.1128/mr.51.1.88-134.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D. A., Bolin J. T., Burridge J. M., Filman D. J., Volz K. W., Kaufman B. T., Beddell C. R., Champness J. N., Stammers D. K., Kraut J. Refined crystal structures of Escherichia coli and chicken liver dihydrofolate reductase containing bound trimethoprim. J Biol Chem. 1985 Jan 10;260(1):381–391. [PubMed] [Google Scholar]
- Rouch D. A., Messerotti L. J., Loo L. S., Jackson C. A., Skurray R. A. Trimethoprim resistance transposon Tn4003 from Staphylococcus aureus encodes genes for a dihydrofolate reductase and thymidylate synthetase flanked by three copies of IS257. Mol Microbiol. 1989 Feb;3(2):161–175. doi: 10.1111/j.1365-2958.1989.tb01805.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. R., Calvo J. M. Nucleotide sequence of the E coli gene coding for dihydrofolate reductase. Nucleic Acids Res. 1980 May 24;8(10):2255–2274. doi: 10.1093/nar/8.10.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stueber D., Ibrahimi I., Cutler D., Dobberstein B., Bujard H. A novel in vitro transcription-translation system: accurate and efficient synthesis of single proteins from cloned DNA sequences. EMBO J. 1984 Dec 20;3(13):3143–3148. doi: 10.1002/j.1460-2075.1984.tb02271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




