Abstract
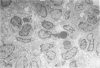
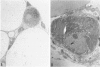
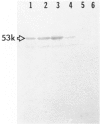
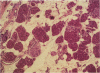
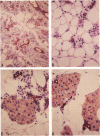
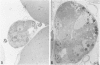
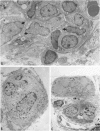
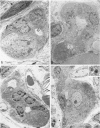
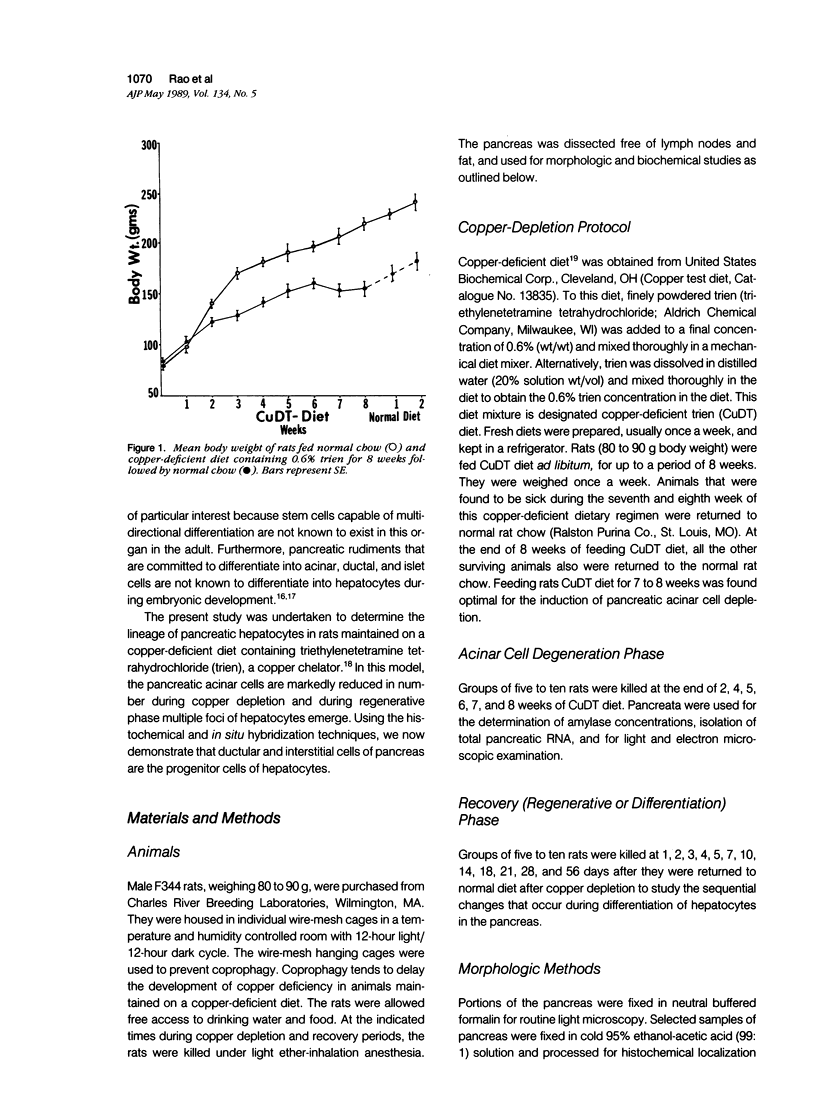
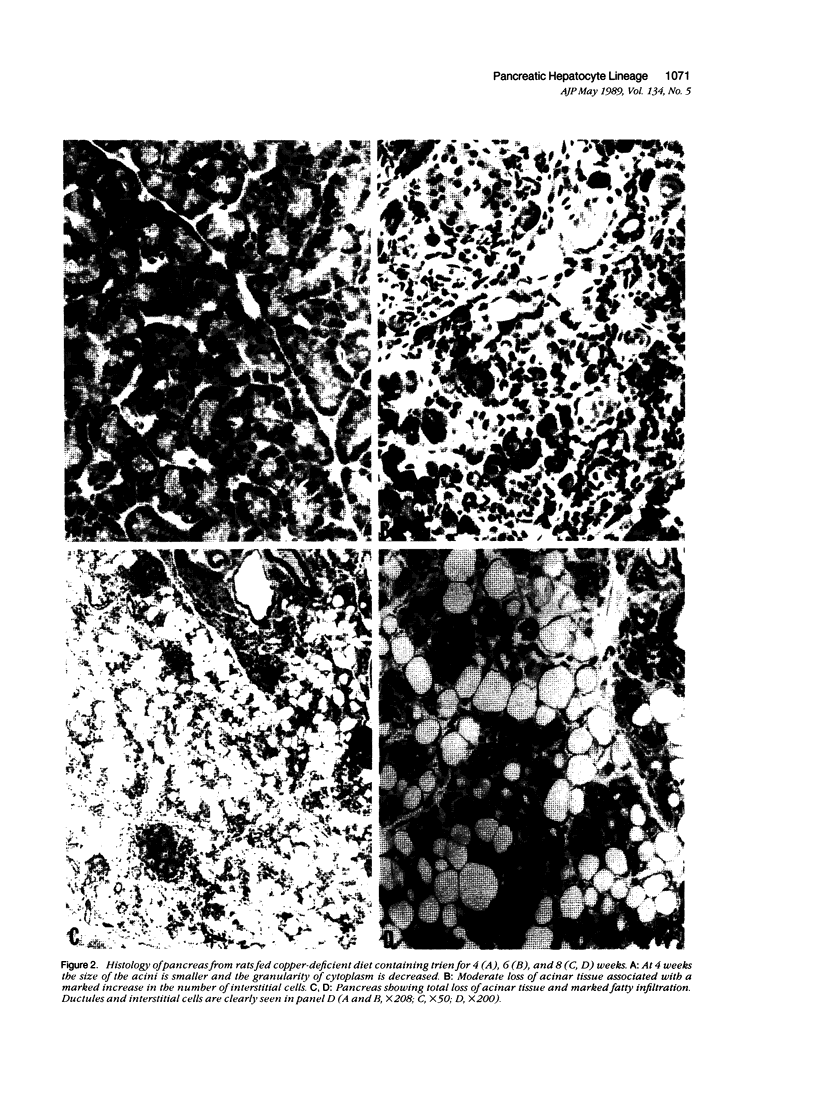
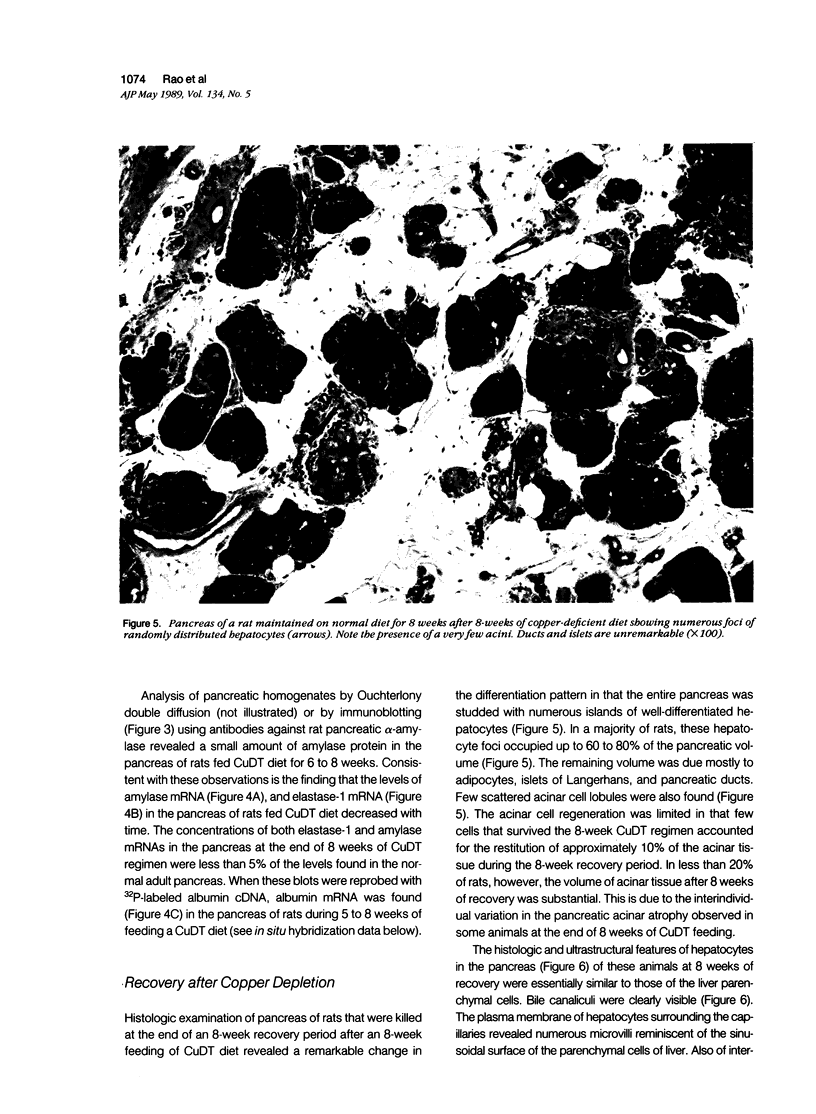
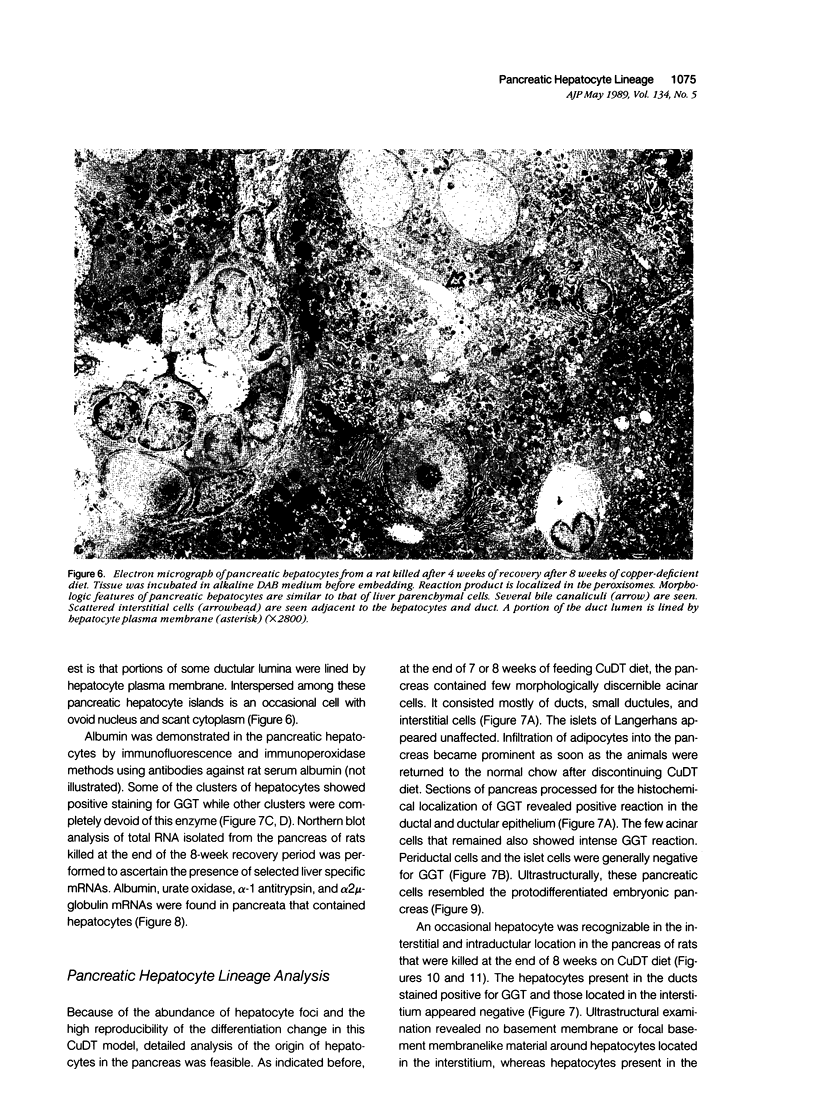
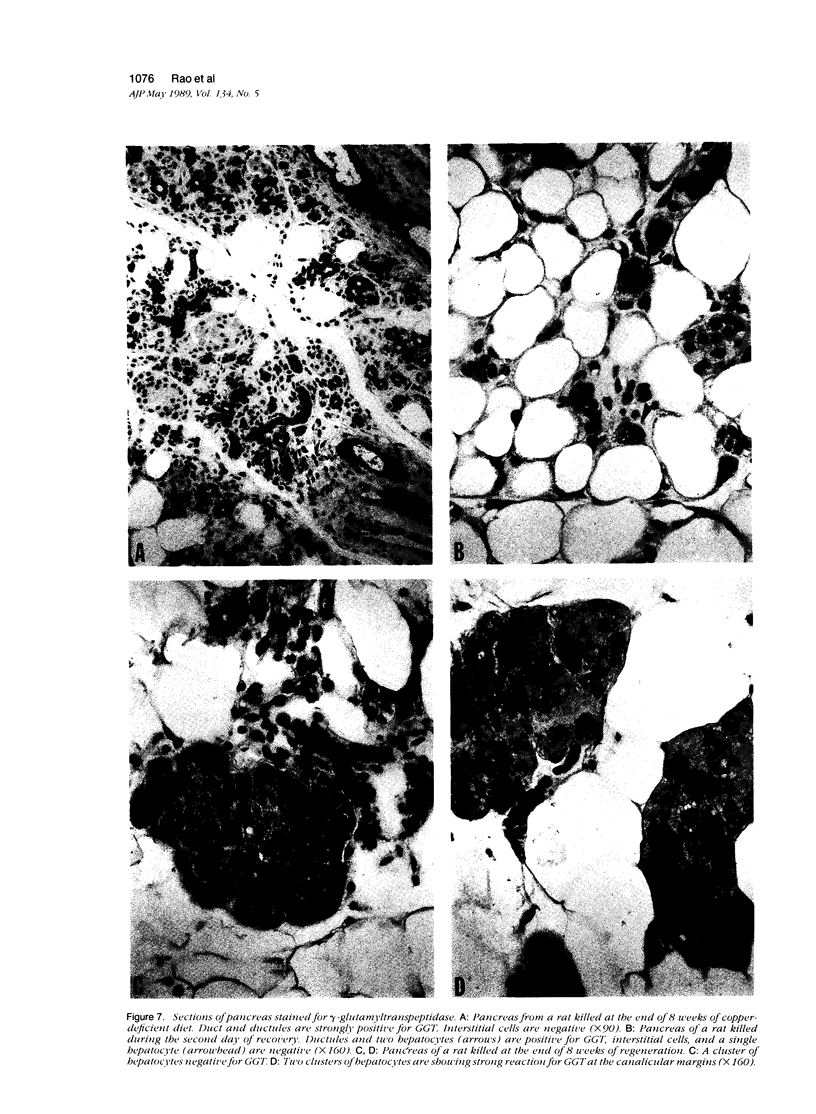
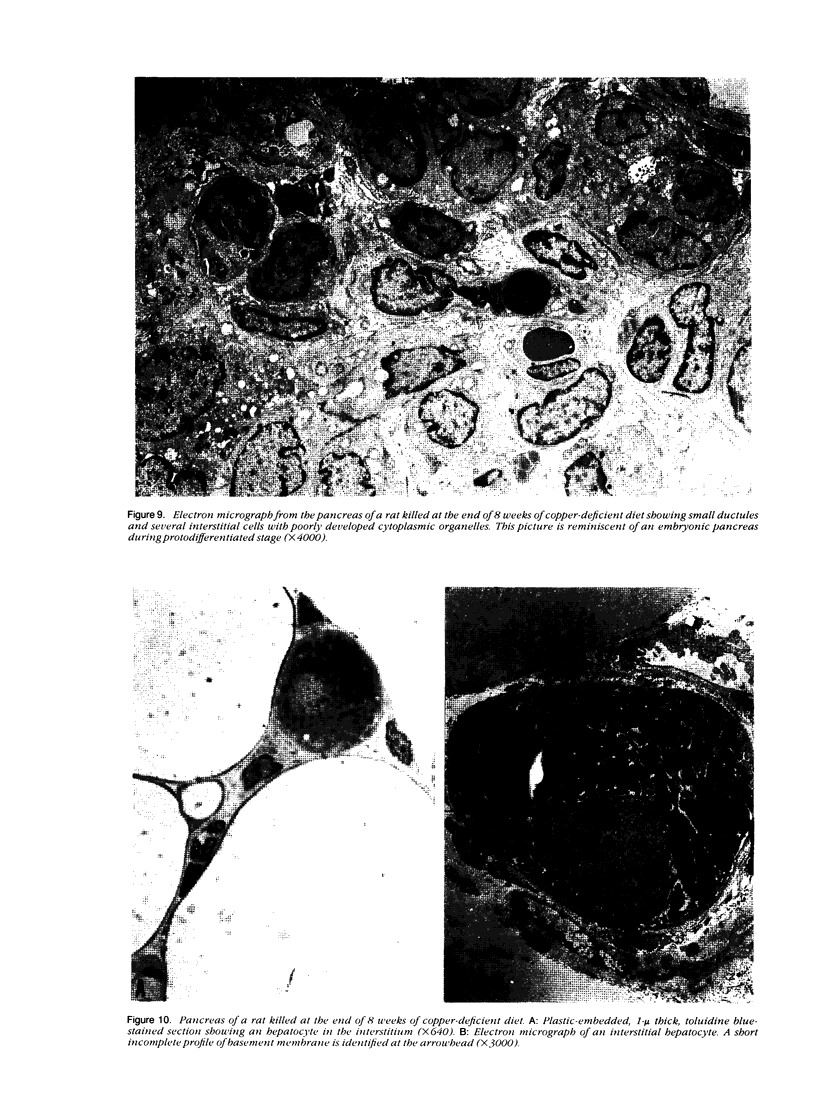
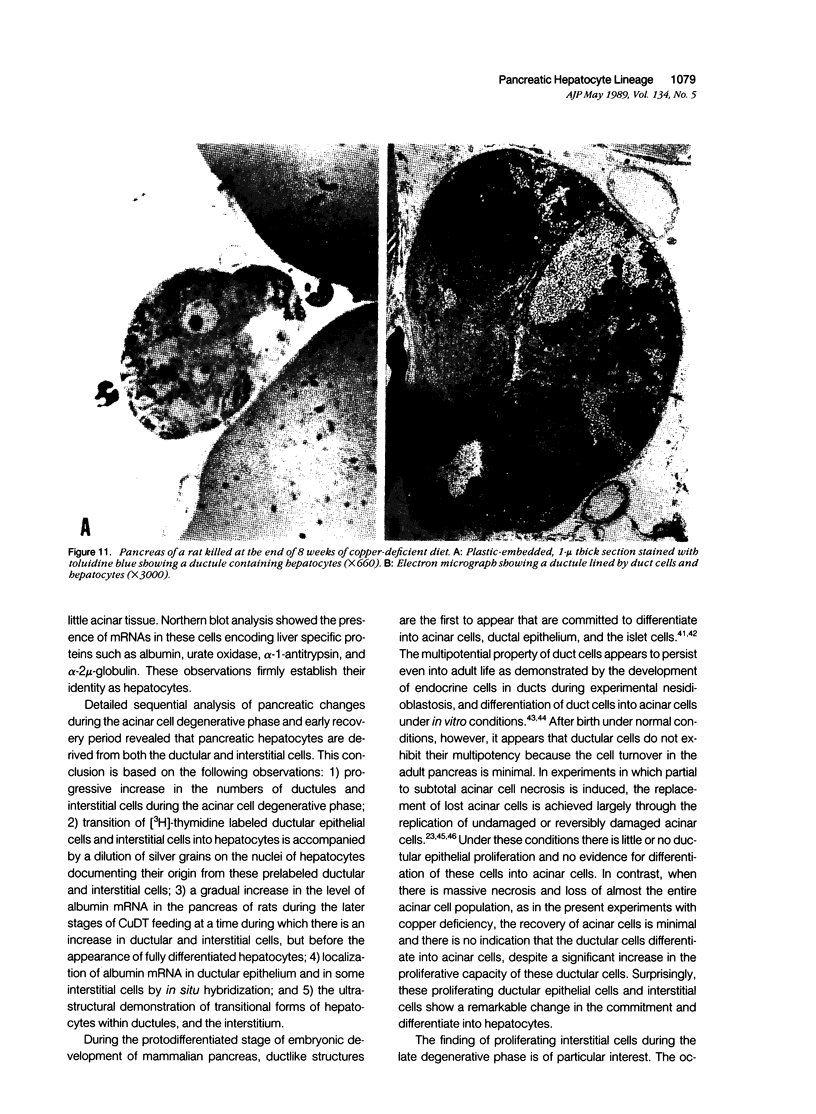
Development of pancreatic hepatocytes in adult rats maintained on copper dificient diet containing 0.6% trien (CuDT) has been reported recently. To elucidate the histogenesis of hepatocytes a sequential study was undertaken using morphologic, histochemical, immunochemical, in situ hybridization, and Northern blot analysis. Male F-344 rats weighing 80 to 90 g were fed CuDT for 8 weeks and returned to normal rat chow. Beginning from 4 weeks of copper depletion, there was a progressive loss of acinar cells and by 8 weeks more than 90% of the acinar tissue was lost. During this period, there was an increase in the number of adipocytes in the interstitium, and in the number of interstitial and ductular cells. Morphologic observations were confirmed by immunoblot and Northern blot analysis, in which the amount of pancreatic proteins and their mRNAs decreased between 5 and 8 weeks. During this period, a progressive increase in the level of albumin mRNA was observed. In situ hybridization, performed at 7 weeks of copper deficiency, showed localization of albumin mRNA over interstitial and ductular cells. Pancreatic hepatocytes were identified immediately after the rats were returned to a normal diet and gradually increased in number. The hepatocytes occupied almost 60% of the pancreatic volume by 8 weeks. During the early recovery phase, hepatocytes were identified in ductules as well as in the interstitium. Based on these studies, it is concluded that both the ductular cells and interstitial cells, which resemble oval cells of liver, are capable of transforming into pancreatic hepatocytes and these cells may be considered stem-cell equivalent.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernuau D., Poliard A., Tournier I., Sala-Trepat J., Feldmann G. All hepatocytes are involved in the expression of the albumin gene in the normal adult rat: a demonstration by in situ hybridization and immunoperoxidase techniques. Cell Biol Int Rep. 1985 Jan;9(1):31–42. doi: 10.1016/0309-1651(85)90139-0. [DOI] [PubMed] [Google Scholar]
- Caplan A. I., Ordahl C. P. Irreversible gene repression model for control of development. Science. 1978 Jul 14;201(4351):120–130. doi: 10.1126/science.351805. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cossel L. Electron microscopic demonstration of intermediate cells in the healthy adult human pancreas. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;52(3):283–287. doi: 10.1007/BF02889969. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- DiBerardino M. A., Hoffner N. J., Etkin L. D. Activation of dormant genes in specialized cells. Science. 1984 Jun 1;224(4652):946–952. doi: 10.1126/science.6719127. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Marsden E., Thorgeirsson S. S. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987 Nov;8(11):1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- FARBER E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956 Feb;16(2):142–148. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fisher M. Neuronal influence on glial enzyme expression: evidence from mutant mouse cerebella. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4414–4418. doi: 10.1073/pnas.81.14.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P. J., Carol B. M., Rosenstock L. Pancreatic acinar cell regeneration. Nature. 1966 Nov 5;212(5062):594–596. doi: 10.1038/212594a0. [DOI] [PubMed] [Google Scholar]
- GRISHAM J. W., HARTROFT W. S. Morphologic identification by electron microscopy of "oval" cells in experimental hepatic degeneration. Lab Invest. 1961 Mar-Apr;10:317–332. [PubMed] [Google Scholar]
- GRISHAM J. W., PORTA E. A. ORIGIN AND FATE OF PROLIFERATED HEPATIC DUCTAL CELLS IN THE RAT: ELECTRON MICROSCOPIC AND AUTORADIOGRAPHIC STUDIES. Exp Mol Pathol. 1964 Jun;86:242–261. doi: 10.1016/0014-4800(64)90057-7. [DOI] [PubMed] [Google Scholar]
- Germain L., Noël M., Gourdeau H., Marceau N. Promotion of growth and differentiation of rat ductular oval cells in primary culture. Cancer Res. 1988 Jan 15;48(2):368–378. [PubMed] [Google Scholar]
- Grisham J. W. Cell types in long-term propagable cultures of rat liver. Ann N Y Acad Sci. 1980;349:128–137. doi: 10.1111/j.1749-6632.1980.tb29521.x. [DOI] [PubMed] [Google Scholar]
- Hansen L. J., Mangkornkanok/Mark M., Reddy J. K. Immunohistochemical localization of pancreatic exocrine enzymes in normal and neoplastic pancreatic acinar epithelium of rat. J Histochem Cytochem. 1981 Feb;29(2):309–313. doi: 10.1177/29.2.6166657. [DOI] [PubMed] [Google Scholar]
- Hayner N. T., Braun L., Yaswen P., Brooks M., Fausto N. Isozyme profiles of oval cells, parenchymal cells, and biliary cells isolated by centrifugal elutriation from normal and preneoplastic livers. Cancer Res. 1984 Jan;44(1):332–338. [PubMed] [Google Scholar]
- Hoover K. L., Poirier L. A. Hepatocyte-like cells within the pancreas of rats fed methyl-deficient diets. J Nutr. 1986 Aug;116(8):1569–1575. doi: 10.1093/jn/116.8.1569. [DOI] [PubMed] [Google Scholar]
- LEBLOND C. P. CLASSIFICATION OF CELL POPULATIONS ON THE BASIS OF THEIR PROLIFERATIVE BEHAVIOR. Natl Cancer Inst Monogr. 1964 May;14:119–150. [PubMed] [Google Scholar]
- LIU H. M., POTTER E. L. Development of the human pancreas. Arch Pathol. 1962 Nov;74:439–452. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lalwani N. D., Reddy M. K., Qureshi S. A., Reddy J. K. Development of hepatocellular carcinomas and increased peroxisomal fatty acid beta-oxidation in rats fed [4-chloro-6-(2,3-xylidino)-2-pyrimidinylthio] acetic acid (Wy-14,643) in the semipurified diet. Carcinogenesis. 1981;2(7):645–650. doi: 10.1093/carcin/2.7.645. [DOI] [PubMed] [Google Scholar]
- Laperche Y., Lynch K. R., Dolan K. P., Feigelson P. Tissue-specific control of alpha 2u globulin gene expression: constitutive synthesis in the submaxillary gland. Cell. 1983 Feb;32(2):453–460. doi: 10.1016/0092-8674(83)90465-8. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Crerar M. M., Swain W. F., Pictet R. L., Thomas G., Rutter W. J. Structure of a family of rat amylase genes. Nature. 1980 Sep 11;287(5778):117–122. doi: 10.1038/287117a0. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Quinto C., Swain W., Pictet R. L., Nikovits W., Rutter W. J. Primary structure of two distinct rat pancreatic preproelastases determined by sequence analysis of the complete cloned messenger ribonucleic acid sequences. Biochemistry. 1982 Mar 16;21(6):1453–1463. doi: 10.1021/bi00535a053. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmed R. N. Intermediate cells of the pancreas. An appraisal. Gastroenterology. 1979 Jan;76(1):196–201. [PubMed] [Google Scholar]
- Moorman A. F., de Boer P. A., Geerts W. J., van den Zande L., Lamers W. H., Charles R. Complementary distribution of carbamoylphosphate synthetase (ammonia) and glutamine synthetase in rat liver acinus is regulated at a pretranslational level. J Histochem Cytochem. 1988 Jul;36(7):751–755. doi: 10.1177/36.7.2898495. [DOI] [PubMed] [Google Scholar]
- Rao M. S., Dwivedi R. S., Subbarao V., Usman M. I., Scarpelli D. G., Nemali M. R., Yeldandi A., Thangada S., Kumar S., Reddy J. K. Almost total conversion of pancreas to liver in the adult rat: a reliable model to study transdifferentiation. Biochem Biophys Res Commun. 1988 Oct 14;156(1):131–136. doi: 10.1016/s0006-291x(88)80814-3. [DOI] [PubMed] [Google Scholar]
- Rao M. S., Reddy M. K., Reddy J. K., Scarpelli D. G. Response of chemically induced hepatocytelike cells in hamster pancreas to methyl clofenapate, a peroxisome proliferator. J Cell Biol. 1982 Oct;95(1):50–56. doi: 10.1083/jcb.95.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M. S., Scarpelli D. G., Reddy J. K. Transdifferentiated hepatocytes in rat pancreas. Curr Top Dev Biol. 1986;20:63–78. doi: 10.1016/s0070-2153(08)60654-7. [DOI] [PubMed] [Google Scholar]
- Rao M. S., Subbarao V., Luetteke N., Scarpelli D. G. Further characterization of carcinogen-induced hepatocytelike cells in hamster pancreas. Am J Pathol. 1983 Jan;110(1):89–94. [PMC free article] [PubMed] [Google Scholar]
- Rao M. S., Subbarao V., Reddy J. K. Induction of hepatocytes in the pancreas of copper-depleted rats following copper repletion. Cell Differ. 1986 Mar;18(2):109–117. doi: 10.1016/0045-6039(86)90005-9. [DOI] [PubMed] [Google Scholar]
- Rao M. S., Subbarao V., Reddy J. K. Peroxisome proliferator-induced hepatocarcinogenesis: histochemical analysis of ciprofibrate-induced preneoplastic and neoplastic lesions for gamma-glutamyl transpeptidase activity. J Natl Cancer Inst. 1986 Oct;77(4):951–956. [PubMed] [Google Scholar]
- Reddy J. K., Rao M. S., Qureshi S. A., Reddy M. K., Scarpelli D. G., Lalwani N. D. Induction and origin of hepatocytes in rat pancreas. J Cell Biol. 1984 Jun;98(6):2082–2090. doi: 10.1083/jcb.98.6.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J. K., Rao M. S., Svoboda D. J., Prasad J. D. Pancreatic necrosis and regeneration induced by 4-hydroxyaminoquinoline-1-oxide in the guinea pig. Lab Invest. 1975 Jan;32(1):98–104. [PubMed] [Google Scholar]
- Reddy P. G., Nemali M. R., Reddy M. K., Reddy M. N., Yuan P. M., Yuen S., Laffler T. G., Shiroza T., Kuramitsu H. K., Usuda N. Isolation and sequence determination of a cDNA clone for rat peroxisomal urate oxidase: liver-specific expression in the rat. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9081–9085. doi: 10.1073/pnas.85.23.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L., Brown R. A., Duguid W. P. A new approach to the induction of duct epithelial hyperplasia and nesidioblastosis by cellophane wrapping of the hamster pancreas. J Surg Res. 1983 Jul;35(1):63–72. doi: 10.1016/0022-4804(83)90127-0. [DOI] [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Rutter W. J., Pictet R. L., Morris P. W. Toward molecular mechanisms of developmental processes. Annu Rev Biochem. 1973;42:601–646. doi: 10.1146/annurev.bi.42.070173.003125. [DOI] [PubMed] [Google Scholar]
- Sarkar B., Sass-Kortsak A., Clarke R., Laurie S. H., Wei P. A comparative study of in vitro and in vivo interaction of D-penicillamine and triethylenetetramine with copper. Proc R Soc Med. 1977;70 (Suppl 3):13–18. doi: 10.1177/00359157770700S307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpelli D. G. Multipotent developmental capacity of cells in the adult animal. Lab Invest. 1985 Apr;52(4):331–333. [PubMed] [Google Scholar]
- Scarpelli D. G., Rao M. S. Differentiation of regenerating pancreatic cells into hepatocyte-like cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2577–2581. doi: 10.1073/pnas.78.4.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpelli D. G., Rao M. S., Reddy J. K. Studies of pancreatic carcinogenesis in different animal models. Environ Health Perspect. 1984 Jun;56:219–227. [PMC free article] [PubMed] [Google Scholar]
- Scarpelli D. G., Rao M. S., Subbarao V., Beversluis M. Regeneration of Syrian golden hamster pancreas and covalent binding of N-nitroso-2,6-[3H]dimethylmorpholine. Cancer Res. 1981 Mar;41(3):1051–1057. [PubMed] [Google Scholar]
- Sell S., Salman J. Light- and electron-microscopic autoradiographic analysis of proliferating cells during the early stages of chemical hepatocarcinogenesis in the rat induced by feeding N-2-fluorenylacetamide in a choline-deficient diet. Am J Pathol. 1984 Feb;114(2):287–300. [PMC free article] [PubMed] [Google Scholar]
- Tatematsu M., Ho R. H., Kaku T., Ekem J. K., Farber E. Studies on the proliferation and fate of oval cells in the liver of rats treated with 2-acetylaminofluorene and partial hepatectomy. Am J Pathol. 1984 Mar;114(3):418–430. [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao M. S., Duguid W. P. Establishment of propagable epithelial cell lines from normal adult rat pancreas. Exp Cell Res. 1987 Feb;168(2):365–375. doi: 10.1016/0014-4827(87)90009-7. [DOI] [PubMed] [Google Scholar]
- Usuda N., Reddy J. K., Hashimoto T., Rao M. S. Immunocytochemical localization of liver-specific proteins in pancreatic hepatocytes of rat. Eur J Cell Biol. 1988 Jun;46(2):299–306. [PubMed] [Google Scholar]
- WILSON J. W., LEDUC E. H. Role of cholangioles in restoration of the liver of the mouse after dietary injury. J Pathol Bacteriol. 1958 Oct;76(2):441–449. doi: 10.1002/path.1700760213. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Evans J. Ultrastructural studies of early morphogenesis and cytodifferentiation in the embryonic mammalian pancreas. Dev Biol. 1968 Apr;17(4):413–446. doi: 10.1016/0012-1606(68)90073-0. [DOI] [PubMed] [Google Scholar]
- Wetts R., Fraser S. E. Multipotent precursors can give rise to all major cell types of the frog retina. Science. 1988 Mar 4;239(4844):1142–1145. doi: 10.1126/science.2449732. [DOI] [PubMed] [Google Scholar]
- Yaswen P., Hayner N. T., Fausto N. Isolation of oval cells by centrifugal elutriation and comparison with other cell types purified from normal and preneoplastic livers. Cancer Res. 1984 Jan;44(1):324–331. [PubMed] [Google Scholar]