Abstract
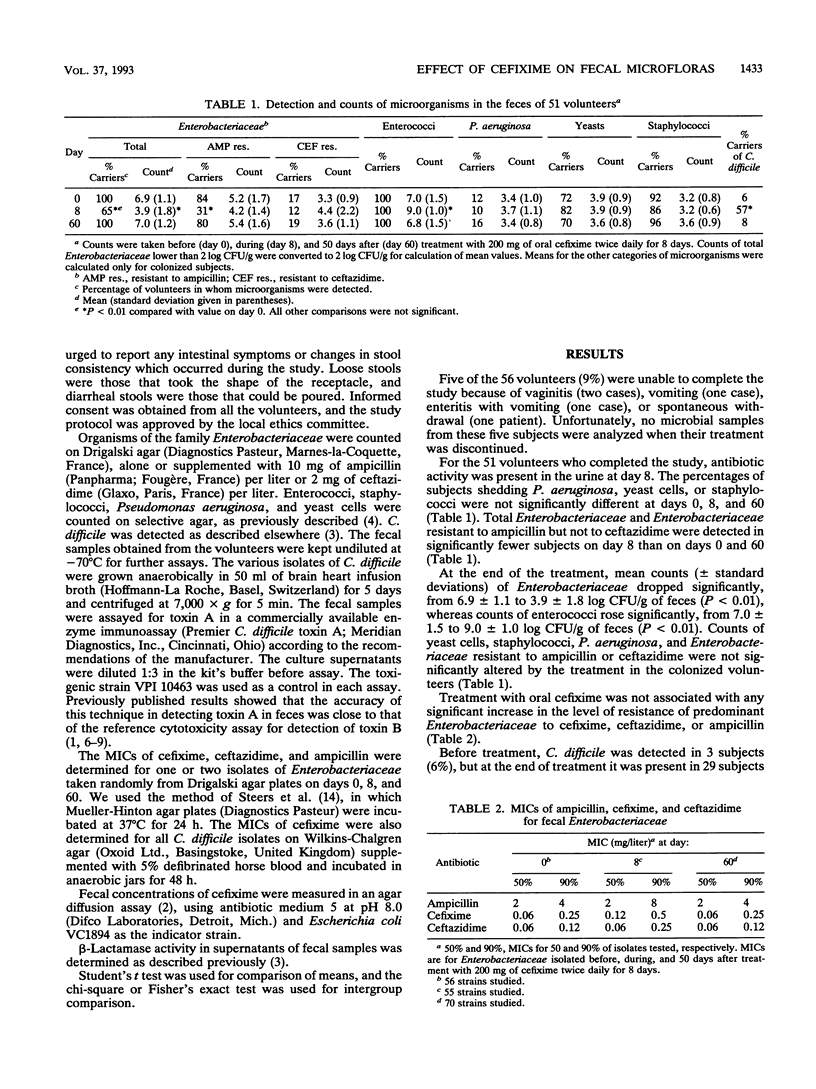
Microbial changes including the shedding of Clostridium difficile, fecal beta-lactamase activity, and gastrointestinal symptoms were assessed in 51 healthy volunteers given 200 mg of cefixime twice daily for 8 days. The number of organisms of the family Enterobacteriaceae (means +/- standard deviations) dropped from 6.9 +/- 1.1 to 3.9 +/- 1.8 log CFU/g of feces (P < 0.01), whereas counts of enterococci rose from 7.0 +/- 1.5 to 9.0 +/- 1.0 log CFU/g of feces (P < 0.01). Both counts returned to their initial levels 50 days after the cessation of treatment. Cefixime did not significantly modify the frequency of fecal excretion of Pseudomonas aeruginosa, Staphylococcus spp., yeasts, or members of the Enterobacteriaceae resistant to ceftazidime or ampicillin. The proportion of subjects shedding C. difficile rose from 6% before treatment to 57% (P < 0.01) at the end of treatment but returned to 8% 50 days thereafter. No case of pseudomembranous colitis was observed. Stool changes occurred in 13 volunteers during treatment (25%) and in 2 others more than 10 days after the end of treatment (4%). These changes were not significantly associated with the shedding of toxigenic strains of C. difficile or with the presence of toxin A in feces. By contrast, during treatment, stool changes occurred in 8 of the 18 volunteers (44%) who had antibiotic activity in their feces but in only 5 of the 33 (15%) for whom no such activity was found (P < 0.05). The absence of antibiotic activity in the feces was itself linked with the presence of beta-lactamase activity in the feces. Since we had found earlier that fecal beta-lactamase activity afforded protection against alteration in stool consistency during treatments with oral cephalosporins, the present study confirmed our previous preliminary results in this respect.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borriello S. P., Vale T., Brazier J. S., Hyde S., Chippeck E. Evaluation of a commercial enzyme immunoassay kit for the detection of Clostridium difficile toxin A. Eur J Clin Microbiol Infect Dis. 1992 Apr;11(4):360–363. doi: 10.1007/BF01962079. [DOI] [PubMed] [Google Scholar]
- CHABBERT Y., BOULINGRE H. Modifications pratiques concernant le dosage des antibiotiques en clinique. Rev Fr Etud Clin Biol. 1957 Jun;2(6):636–640. [PubMed] [Google Scholar]
- Chachaty E., Depitre C., Mario N., Bourneix C., Saulnier P., Corthier G., Andremont A. Presence of Clostridium difficile and antibiotic and beta-lactamase activities in feces of volunteers treated with oral cefixime, oral cefpodoxime proxetil, or placebo. Antimicrob Agents Chemother. 1992 Sep;36(9):2009–2013. doi: 10.1128/aac.36.9.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen M. R., Bonnén H., Tvede M., Mortensen P. B. Colonic fermentation to short-chain fatty acids is decreased in antibiotic-associated diarrhea. Gastroenterology. 1991 Dec;101(6):1497–1504. doi: 10.1016/0016-5085(91)90384-w. [DOI] [PubMed] [Google Scholar]
- De Girolami P. C., Hanff P. A., Eichelberger K., Longhi L., Teresa H., Pratt J., Cheng A., Letourneau J. M., Thorne G. M. Multicenter evaluation of a new enzyme immunoassay for detection of Clostridium difficile enterotoxin A. J Clin Microbiol. 1992 May;30(5):1085–1088. doi: 10.1128/jcm.30.5.1085-1088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmée M., Mackey T., Hamitou A. Evaluation of a new commercial Clostridium difficile toxin A enzyme immunoassay using diarrhoeal stools. Eur J Clin Microbiol Infect Dis. 1992 Mar;11(3):246–249. doi: 10.1007/BF02098089. [DOI] [PubMed] [Google Scholar]
- DiPersio J. R., Varga F. J., Conwell D. L., Kraft J. A., Kozak K. J., Willis D. H. Development of a rapid enzyme immunoassay for Clostridium difficile toxin A and its use in the diagnosis of C. difficile-associated disease. J Clin Microbiol. 1991 Dec;29(12):2724–2730. doi: 10.1128/jcm.29.12.2724-2730.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doern G. V., Coughlin R. T., Wu L. Laboratory diagnosis of Clostridium difficile-associated gastrointestinal disease: comparison of a monoclonal antibody enzyme immunoassay for toxins A and B with a monoclonal antibody enzyme immunoassay for toxin A only and two cytotoxicity assays. J Clin Microbiol. 1992 Aug;30(8):2042–2046. doi: 10.1128/jcm.30.8.2042-2046.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp C. C., Sierra-Madero J., Washington J. A. Antibacterial activities of cefpodoxime, cefixime, and ceftriaxone. Antimicrob Agents Chemother. 1988 Dec;32(12):1896–1898. doi: 10.1128/aac.32.12.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léonard F., Andremont A., Leclerq B., Labia R., Tancrède C. Use of beta-lactamase-producing anaerobes to prevent ceftriaxone from degrading intestinal resistance to colonization. J Infect Dis. 1989 Aug;160(2):274–280. doi: 10.1093/infdis/160.2.274. [DOI] [PubMed] [Google Scholar]
- Rao S. S., Edwards C. A., Austen C. J., Bruce C., Read N. W. Impaired colonic fermentation of carbohydrate after ampicillin. Gastroenterology. 1988 Apr;94(4):928–932. doi: 10.1016/0016-5085(88)90549-5. [DOI] [PubMed] [Google Scholar]
- Rolfe R. D., Finegold S. M. Intestinal beta-lactamase activity in ampicillin-induced, Clostridium difficile-associated ileocecitis. J Infect Dis. 1983 Feb;147(2):227–235. doi: 10.1093/infdis/147.2.227. [DOI] [PubMed] [Google Scholar]
- Wise R. The pharmacokinetics of the oral cephalosporins--a review. J Antimicrob Chemother. 1990 Dec;26 (Suppl E):13–20. doi: 10.1093/jac/26.suppl_e.13. [DOI] [PubMed] [Google Scholar]



