Abstract
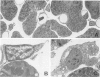
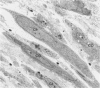
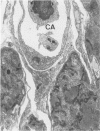
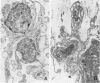
To better understand the tendency of myocardium to heal by scarring rather than regeneration, the authors examined the responses of connective tissue cells (CTCs) after three types of necrotizing injuries. Derived from myocardial interstitial cells, CTCs proliferated in both the connective tissue space and the compartment of necrotic myocytes. They assumed various cell forms: fibrocytelike CTCs throughout the sites of injury deposited extracellular scar tissue elements, established CTC-myocyte contacts, and helped anchor myocytes to scar tissue with myotendonlike specializations; CTCs with more complex forms established CTC-myocyte relationships, suggesting important roles in communication and tissue remodeling. CTCs within scar tissue differentiated into myofibrocytes, chondrocytes, and possibly smooth muscle cells. Most scar tissue elements were disposed in the long axis of myocytes. These alterations in form indicate that CTCs have various roles in myocardial repair and suggest that a number of the roles are modulated by contractile forces.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bluemink J. G., Van Maurik P., Lawson K. A. Intimate cell contacts at the epithelial/mesenchymal interface in embryonic mouse lung. J Ultrastruct Res. 1976 May;55(2):257–270. doi: 10.1016/s0022-5320(76)80071-8. [DOI] [PubMed] [Google Scholar]
- Brody J. S., Vaccaro C. A., Gill P. J., Silbert J. E. Alterations in alveolar basement membranes during postnatal lung growth. J Cell Biol. 1982 Nov;95(2 Pt 1):394–402. doi: 10.1083/jcb.95.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancilla P. A., Frommes S. P., Kahn L. E., DeBault L. E. Regeneration of cerebral microvessels: a morphologic and histochemical study after local freeze-injury. Lab Invest. 1979 Jan;40(1):74–82. [PubMed] [Google Scholar]
- Cantin M., Ballak M., Beuzeron-Mangina J., Anand-Srivastava M. B., Tautu C. DNA synthesis in cultured adult cardiocytes. Science. 1981 Oct 30;214(4520):569–570. doi: 10.1126/science.7291996. [DOI] [PubMed] [Google Scholar]
- Claycomb W. C. Long-term culture and characterization of the adult ventricular and atrial cardiac muscle cell. Basic Res Cardiol. 1985;80 (Suppl 2):171–174. [PubMed] [Google Scholar]
- Cutler L. S., Chaudhry A. P. Intercellular contacts at the epithelial-mesenchymal interface during the prenatal development of the rat submandibular gland. Dev Biol. 1973 Aug;33(2):229–240. doi: 10.1016/0012-1606(73)90133-4. [DOI] [PubMed] [Google Scholar]
- Fishbein M. C., Maclean D., Maroko P. R. The histopathologic evolution of myocardial infarction. Chest. 1978 Jun;73(6):843–849. doi: 10.1378/chest.73.6.843. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Hirschel B. J., Ryan G. B., Statkov P. R., Majno G. Granulation tissue as a contractile organ. A study of structure and function. J Exp Med. 1972 Apr 1;135(4):719–734. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobstein C. Mechanisms of organogenetic tissue interaction. Natl Cancer Inst Monogr. 1967 Sep;26:279–299. [PubMed] [Google Scholar]
- Hanak H., Böck P. Die Feinstruktur der Muskel-Sehnenverbindung von Skelett- und Herzmuskel. J Ultrastruct Res. 1971 Jul;36(1):68–85. doi: 10.1016/s0022-5320(71)80089-8. [DOI] [PubMed] [Google Scholar]
- Hardy M. H., Goldberg E. A. Morphological changes at the basement membrane during some tissue interactions in the integument. Can J Biochem Cell Biol. 1983 Aug;61(8):957–966. doi: 10.1139/o83-122. [DOI] [PubMed] [Google Scholar]
- Haston W. S., Shields J. M., Wilkinson P. C. The orientation of fibroblasts and neutrophils on elastic substrata. Exp Cell Res. 1983 Jun;146(1):117–126. doi: 10.1016/0014-4827(83)90330-0. [DOI] [PubMed] [Google Scholar]
- Jugdutt B. I., Amy R. W. Healing after myocardial infarction in the dog: changes in infarct hydroxyproline and topography. J Am Coll Cardiol. 1986 Jan;7(1):91–102. doi: 10.1016/s0735-1097(86)80265-0. [DOI] [PubMed] [Google Scholar]
- Kollros P. R., Bates S. R., Mathews M. B., Horwitz A. L., Glagov S. Cyclic AMP inhibits increased collagen production by cyclically stretched smooth muscle cells. Lab Invest. 1987 Apr;56(4):410–417. [PubMed] [Google Scholar]
- Mathan M., Hermos J. A., Trier J. S. Structural features of the epithelio-mesenchymal interface of rat duodenal mucosa during development. J Cell Biol. 1972 Mar;52(3):577–588. doi: 10.1083/jcb.52.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallister L. P., Page E. Effects of thyroxin on ultrastructure of rat myocardial cells: a stereological study. J Ultrastruct Res. 1973 Jan;42(1):136–155. doi: 10.1016/s0022-5320(73)80012-7. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Nag A. C., Cheng M. Adult mammalian cardiac muscle cells in culture. Tissue Cell. 1981;13(3):515–523. doi: 10.1016/0040-8166(81)90023-9. [DOI] [PubMed] [Google Scholar]
- Nag A. C., Cheng M. DNA synthesis of adult mammalian cardiac muscle cells in long-term culture. Tissue Cell. 1986;18(4):491–497. doi: 10.1016/0040-8166(86)90015-7. [DOI] [PubMed] [Google Scholar]
- OLIVER J. Correlations of structure and function and mechanisms of recovery in acute tubular necrosis. Am J Med. 1953 Oct;15(4):535–557. doi: 10.1016/0002-9343(53)90143-0. [DOI] [PubMed] [Google Scholar]
- RING P. A. Myocardial regeneration in experimental ischaemic lesions of the heart. J Pathol Bacteriol. 1950 Jan;62(1):21–27. doi: 10.1002/path.1700620103. [DOI] [PubMed] [Google Scholar]
- RONA G., KAHN D. S., CHAPPEL C. I. Study on the healing of cardiac necrosis in the rat. Am J Pathol. 1961 Oct;39:473–489. [PMC free article] [PubMed] [Google Scholar]
- Ross R. The fibroblast and wound repair. Biol Rev Camb Philos Soc. 1968 Feb;43(1):51–96. doi: 10.1111/j.1469-185x.1968.tb01109.x. [DOI] [PubMed] [Google Scholar]
- Rumyantsev P. P. Interrelations of the proliferation and differentiation processes during cardiact myogenesis and regeneration. Int Rev Cytol. 1977;51:186–273. [PubMed] [Google Scholar]
- SELYE H., BAJUSZ E., GRASSO S., MENDELL P. Simple techniques for the surgical occlusion of coronary vessels in the rat. Angiology. 1960 Oct;11:398–407. doi: 10.1177/000331976001100505. [DOI] [PubMed] [Google Scholar]
- Schippel K., Reissig D. Uber die Feinstruktur der Befestigung der Chorda tendinea an der Herzmuskulatur. Z Mikrosk Anat Forsch. 1966;75(1):210–223. [PubMed] [Google Scholar]
- Schmalbruch H. The morphology of regeneration of skeletal muscles in the rat. Tissue Cell. 1976;8(4):673–692. doi: 10.1016/0040-8166(76)90039-2. [DOI] [PubMed] [Google Scholar]
- Thorning D., Vracko R. Renal glomerular basal lamina scaffold: embryologic development, anatomy, and role in cellular reconstruction of rat glomeruli injured by freezing and thawing. Lab Invest. 1977 Jul;37(1):105–119. [PubMed] [Google Scholar]
- Tsukada T., McNutt M. A., Ross R., Gown A. M. HHF35, a muscle actin-specific monoclonal antibody. II. Reactivity in normal, reactive, and neoplastic human tissues. Am J Pathol. 1987 May;127(2):389–402. [PMC free article] [PubMed] [Google Scholar]
- Ursell P. C., Gardner P. I., Albala A., Fenoglio J. J., Jr, Wit A. L. Structural and electrophysiological changes in the epicardial border zone of canine myocardial infarcts during infarct healing. Circ Res. 1985 Mar;56(3):436–451. doi: 10.1161/01.res.56.3.436. [DOI] [PubMed] [Google Scholar]
- Vandenburgh H., Kaufman S. In vitro model for stretch-induced hypertrophy of skeletal muscle. Science. 1979 Jan 19;203(4377):265–268. doi: 10.1126/science.569901. [DOI] [PubMed] [Google Scholar]
- Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974 Nov;77(2):314–346. [PMC free article] [PubMed] [Google Scholar]
- Vracko R., Benditt E. P. Basal lamina: the scaffold for orderly cell replacement. Observations on regeneration of injured skeletal muscle fibers and capillaries. J Cell Biol. 1972 Nov;55(2):406–419. doi: 10.1083/jcb.55.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vracko R., Cunningham D., Frederickson R. G., Thorning D. Basal lamina of rat myocardium. Its fate after death of cardiac myocytes. Lab Invest. 1988 Jan;58(1):77–87. [PubMed] [Google Scholar]
- Vracko R. Significance of basal lamina for regeneration of injured lung. Virchows Arch A Pathol Pathol Anat. 1972;355(3):264–274. doi: 10.1007/BF00551062. [DOI] [PubMed] [Google Scholar]
- Vracko R., Thorning D., Frederickson R. G., Cunningham D. Myocyte reactions at the borders of injured and healing rat myocardium. Lab Invest. 1988 Jul;59(1):104–114. [PubMed] [Google Scholar]
- Vracko R., Thorning D. Freeze-thaw injury of rat heart across an intact diaphragm: a new model for the study of the response of myocardium to injury. Cardiovasc Res. 1985 Feb;19(2):76–84. doi: 10.1093/cvr/19.2.76. [DOI] [PubMed] [Google Scholar]