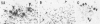Abstract
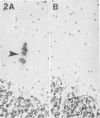
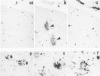
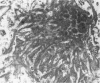
Diffuse senile plaques are characterized by the presence of beta protein (beta P), also called A4 protein, in a dispersed form and the apparent lack of associated dystrophic neurites or reactive glial cells. They are the most common type of senile plaque found in the cerebral cortex in Alzheimer's disease (AD), Down's syndrome (DS), and normal aging. Here is reported the frequent presence of diffuse senile plaques in the molecular layer of cerebellar cortex in AD. Typical neuritic plaques were never detected in this location, making the cerebellar molecular cortex a useful site for the study of diffuse plaques because diffuse plaques in the cerebral cortex are intermingled with neuritic plaques. Diffuse cerebellar plaques were detected by modified Bielschowsky silver stain in 47 of 100 cases of clinically and pathologically diagnosed AD and in none of 40 aged demented and nondemented controls. They were immunolabeled by antibodies to purified AD meningeal or cortical beta P, and to a synthetic beta P but not by two antibodies to the carboxyl- and amino-termini of the beta protein precursor (beta PP), which label a subgroup of cerebral cortical plaques. This latter result suggests that the beta P deposited in the cerebellar molecular layer may be derived from a form of the beta PP from which the carboxyl and amino terminal regions of the precursor have already been cleaved. Diffuse cerebellar plaques were not recognized by antibodies to neurofilaments, tau, and PHF, all of which detect dystrophic neurites in cerebral cortical neuritic plaques. Also, no association of reactive astrocytes or microglial cells with diffuse cerebellar plaques was observed. Thus, diffuse cerebellar plaques represent multifocal deposits of noncompacted beta P that cause little or no morphologic reaction in their microenvironment.
Full text
PDF






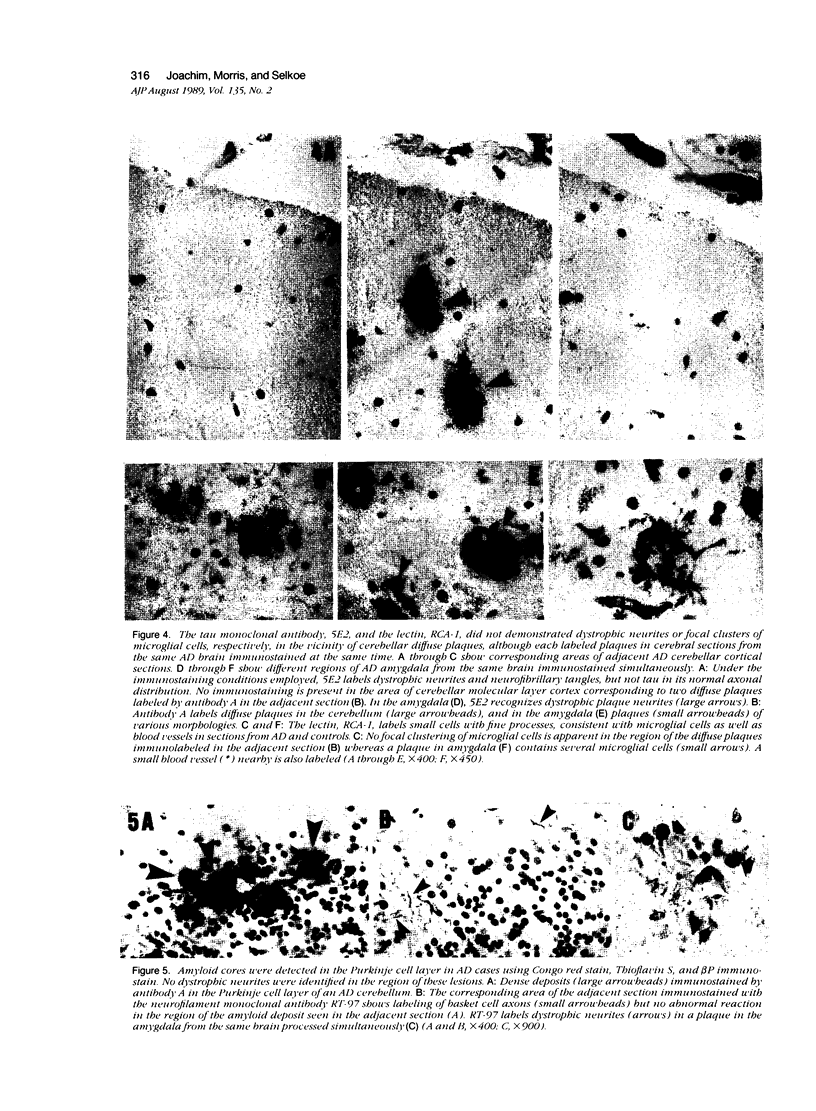



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton B. H., Breinburg D., Downes M. J., Green P. J., Tomlinson B. E., Ulrich J., Wood J. N., Kahn J. Monoclonal antibodies show that neurofibrillary tangles and neurofilaments share antigenic determinants. Nature. 1982 Jul 1;298(5869):84–86. doi: 10.1038/298084a0. [DOI] [PubMed] [Google Scholar]
- Bahmanyar S., Higgins G. A., Goldgaber D., Lewis D. A., Morrison J. H., Wilson M. C., Shankar S. K., Gajdusek D. C. Localization of amyloid beta protein messenger RNA in brains from patients with Alzheimer's disease. Science. 1987 Jul 3;237(4810):77–80. doi: 10.1126/science.3299701. [DOI] [PubMed] [Google Scholar]
- Cohen M. L., Golde T. E., Usiak M. F., Younkin L. H., Younkin S. G. In situ hybridization of nucleus basalis neurons shows increased beta-amyloid mRNA in Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1227–1231. doi: 10.1073/pnas.85.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies L., Wolska B., Hilbich C., Multhaup G., Martins R., Simms G., Beyreuther K., Masters C. L. A4 amyloid protein deposition and the diagnosis of Alzheimer's disease: prevalence in aged brains determined by immunocytochemistry compared with conventional neuropathologic techniques. Neurology. 1988 Nov;38(11):1688–1693. doi: 10.1212/wnl.38.11.1688. [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Farlo J., Davies P., Crystal H., Fuld P., Yen S. H. Alzheimer's disease. A double-labeling immunohistochemical study of senile plaques. Am J Pathol. 1988 Jul;132(1):86–101. [PMC free article] [PubMed] [Google Scholar]
- Ihara Y., Abraham C., Selkoe D. J. Antibodies to paired helical filaments in Alzheimer's disease do not recognize normal brain proteins. Nature. 1983 Aug 25;304(5928):727–730. doi: 10.1038/304727a0. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Duffy L. K., Morris J. H., Selkoe D. J. Protein chemical and immunocytochemical studies of meningovascular beta-amyloid protein in Alzheimer's disease and normal aging. Brain Res. 1988 Nov 22;474(1):100–111. doi: 10.1016/0006-8993(88)90673-7. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Morris J. H., Kosik K. S., Selkoe D. J. Tau antisera recognize neurofibrillary tangles in a range of neurodegenerative disorders. Ann Neurol. 1987 Oct;22(4):514–520. doi: 10.1002/ana.410220411. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Morris J. H., Selkoe D. J. Clinically diagnosed Alzheimer's disease: autopsy results in 150 cases. Ann Neurol. 1988 Jul;24(1):50–56. doi: 10.1002/ana.410240110. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Morris J. H., Selkoe D. J., Kosik K. S. Tau epitopes are incorporated into a range of lesions in Alzheimer's disease. J Neuropathol Exp Neurol. 1987 Nov;46(6):611–622. doi: 10.1097/00005072-198711000-00001. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Ogomori K., Tateishi J., Prusiner S. B. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest. 1987 Aug;57(2):230–236. [PubMed] [Google Scholar]
- Palmert M. R., Podlisny M. B., Witker D. S., Oltersdorf T., Younkin L. H., Selkoe D. J., Younkin S. G. Antisera to an amino-terminal peptide detect the amyloid protein precursor of Alzheimer's disease and recognize senile plaques. Biochem Biophys Res Commun. 1988 Oct 14;156(1):432–437. doi: 10.1016/s0006-291x(88)80859-3. [DOI] [PubMed] [Google Scholar]
- Pro J. D., Smith C. H., Sumi S. M. Presenile Alzheimer disease: amyloid plaques in the cerebellum. Neurology. 1980 Aug;30(8):820–825. doi: 10.1212/wnl.30.8.820. [DOI] [PubMed] [Google Scholar]
- Rasool C. G., Abraham C., Anderton B. H., Haugh M., Kahn J., Selkoe D. J. Alzheimer's disease: immunoreactivity of neurofibrillary tangles with anti-neurofilament and anti-paired helical filament antibodies. Brain Res. 1984 Sep 24;310(2):249–260. doi: 10.1016/0006-8993(84)90148-3. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Abraham C. R., Podlisny M. B., Duffy L. K. Isolation of low-molecular-weight proteins from amyloid plaque fibers in Alzheimer's disease. J Neurochem. 1986 Jun;46(6):1820–1834. doi: 10.1111/j.1471-4159.1986.tb08501.x. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Bell D. S., Podlisny M. B., Price D. L., Cork L. C. Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer's disease. Science. 1987 Feb 20;235(4791):873–877. doi: 10.1126/science.3544219. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Podlisny M. B., Joachim C. L., Vickers E. A., Lee G., Fritz L. C., Oltersdorf T. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich J. Alzheimer changes in nondemented patients younger than sixty-five: possible early stages of Alzheimer's disease and senile dementia of Alzheimer type. Ann Neurol. 1985 Mar;17(3):273–277. doi: 10.1002/ana.410170309. [DOI] [PubMed] [Google Scholar]
- Wong C. W., Quaranta V., Glenner G. G. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]