Abstract
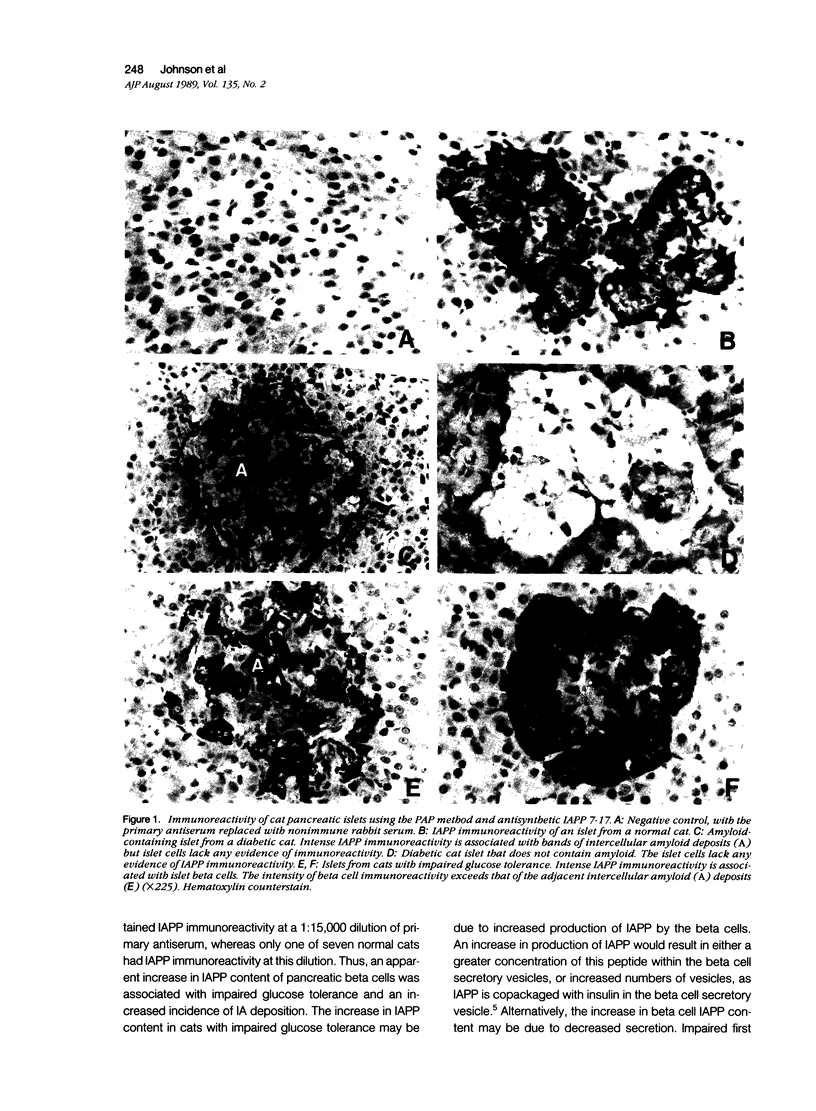
Adult cats determined by clinical laboratory evaluations to be normal, impaired glucose tolerant, or overtly diabetic were used to explore prospectively the relationships among pancreatic beta cell islet amyloid polypeptide (IAPP) immunoreactivity, islet amyloid (IA) deposition, and diabetogenesis. IAPP-derived IA was found in 11 of 14 (79%) diabetic cats, in four of nine (44%) impaired glucose tolerant cats, and in two of eight (25%) normal adult cats. The presence of IA even in very small amounts, therefore, predicts a very high probability (88%) that an animal has either impaired glucose tolerance or overt DM. Although all overtly diabetic cats had a marked decrease or absence of beta cell IAPP immunoreactivity, six of six cats with impaired glucose tolerance retained IAPP immunoreactivity with 1:15,000 dilutions of antisynthetic IAPP 7-17, whereas only one of seven normal cats had IAPP immunoreactivity beyond 1:10,000 dilutions. These findings suggest that increased IAPP production preceding the development of overt DM is linked to the progressive formation of insoluble IA deposits that are apparent in most overtly diabetic individuals. Of most importance, in that IAPP has been reported to inhibit both basal and insulin-stimulated rates of glycogen synthesis, is the possibility that increased production and release of IAPP by pancreatic beta cells plays a key role in the development of the insulin resistance and impaired glucose tolerance, both of which occur in Type 2 DM.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Cooper G. J., Leighton B., Dimitriadis G. D., Parry-Billings M., Kowalchuk J. M., Howland K., Rothbard J. B., Willis A. C., Reid K. B. Amylin found in amyloid deposits in human type 2 diabetes mellitus may be a hormone that regulates glycogen metabolism in skeletal muscle. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7763–7766. doi: 10.1073/pnas.85.20.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. J., Willis A. C., Clark A., Turner R. C., Sim R. B., Reid K. B. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHRLICH J. C., RATNER I. M. Amyloidosis of the islets of Langerhans. A restudy of islet hyalin in diabetic and non-diabetic individuals. Am J Pathol. 1961 Jan;38:49–59. [PMC free article] [PubMed] [Google Scholar]
- Erlandsen S. L., Parsons J. A., Burke J. P., Redick J. A., Van Orden D. E., Van Orden L. S. A modification of the unlabeled antibody enzyme method using heterologous antisera for the light microscopic and ultrastructural localization of insulin, glucagon and growth hormone. J Histochem Cytochem. 1975 Sep;23(9):666–677. doi: 10.1177/23.9.1176760. [DOI] [PubMed] [Google Scholar]
- Howard C. F., Jr Insular amyloidosis and diabetes mellitus in Macaca nigra. Diabetes. 1978 Apr;27(4):357–364. doi: 10.2337/diab.27.4.357. [DOI] [PubMed] [Google Scholar]
- Johnson K. H., Hayden D. W., O'Brien T. D., Westermark P. Spontaneous diabetes mellitus-islet amyloid complex in adult cats. Am J Pathol. 1986 Nov;125(2):416–419. [PMC free article] [PubMed] [Google Scholar]
- Leighton B., Cooper G. J. Pancreatic amylin and calcitonin gene-related peptide cause resistance to insulin in skeletal muscle in vitro. Nature. 1988 Oct 13;335(6191):632–635. doi: 10.1038/335632a0. [DOI] [PubMed] [Google Scholar]
- O'Brien T. D., Hayden D. W., Johnson K. H., Fletcher T. F. Immunohistochemical morphometry of pancreatic endocrine cells in diabetic, normoglycaemic glucose-intolerant and normal cats. J Comp Pathol. 1986 Jul;96(4):357–369. doi: 10.1016/0021-9975(86)90031-9. [DOI] [PubMed] [Google Scholar]
- O'Brien T. D., Hayden D. W., Johnson K. H., Stevens J. B. High dose intravenous glucose tolerance test and serum insulin and glucagon levels in diabetic and non-diabetic cats: relationships to insular amyloidosis. Vet Pathol. 1985 May;22(3):250–261. doi: 10.1177/030098588502200308. [DOI] [PubMed] [Google Scholar]
- Sanke T., Bell G. I., Sample C., Rubenstein A. H., Steiner D. F. An islet amyloid peptide is derived from an 89-amino acid precursor by proteolytic processing. J Biol Chem. 1988 Nov 25;263(33):17243–17246. [PubMed] [Google Scholar]
- Steenbergh P. H., Höppener J. W., Zandberg J., Lips C. J., Jansz H. S. A second human calcitonin/CGRP gene. FEBS Lett. 1985 Apr 22;183(2):403–407. doi: 10.1016/0014-5793(85)80820-6. [DOI] [PubMed] [Google Scholar]
- Westermark P., Grimelius L. The pancreatic islet cells in insular amyloidosis in human diabetic and non-diabetic adults. Acta Pathol Microbiol Scand A. 1973 May;81(3):291–300. doi: 10.1111/j.1699-0463.1973.tb03538.x. [DOI] [PubMed] [Google Scholar]
- Westermark P., Johnson K. H. The pathogenesis of maturity-onset diabetes mellitus: is there a link to islet amyloid polypeptide? Bioessays. 1988 Jul;9(1):30–33. doi: 10.1002/bies.950090109. [DOI] [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., O'Brien T. D., Hayden D. W., Johnson K. H. Islet amyloid in type 2 human diabetes mellitus and adult diabetic cats contains a novel putative polypeptide hormone. Am J Pathol. 1987 Jun;127(3):414–417. [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., Wilander E., Hayden D. W., O'Brien T. D., Johnson K. H. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Wilander E. The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia. 1978 Nov;15(5):417–421. doi: 10.1007/BF01219652. [DOI] [PubMed] [Google Scholar]
- Westermark P., Wilander E., Westermark G. T., Johnson K. H. Islet amyloid polypeptide-like immunoreactivity in the islet B cells of type 2 (non-insulin-dependent) diabetic and non-diabetic individuals. Diabetologia. 1987 Nov;30(11):887–892. doi: 10.1007/BF00274799. [DOI] [PubMed] [Google Scholar]
- Yano B. L., Hayden D. W., Johnson K. H. Feline insular amyloid: association with diabetes mellitus. Vet Pathol. 1981 Sep;18(5):621–627. doi: 10.1177/030098588101800507. [DOI] [PubMed] [Google Scholar]
- Yano B. L., Hayden D. W., Johnson K. H. Feline insular amyloid: incidence in adult cats with no clinicopathologic evidence of overt diabetes mellitus. Vet Pathol. 1981 May;18(3):310–315. doi: 10.1177/030098588101800303. [DOI] [PubMed] [Google Scholar]