Abstract
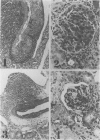
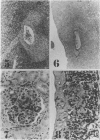
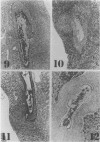
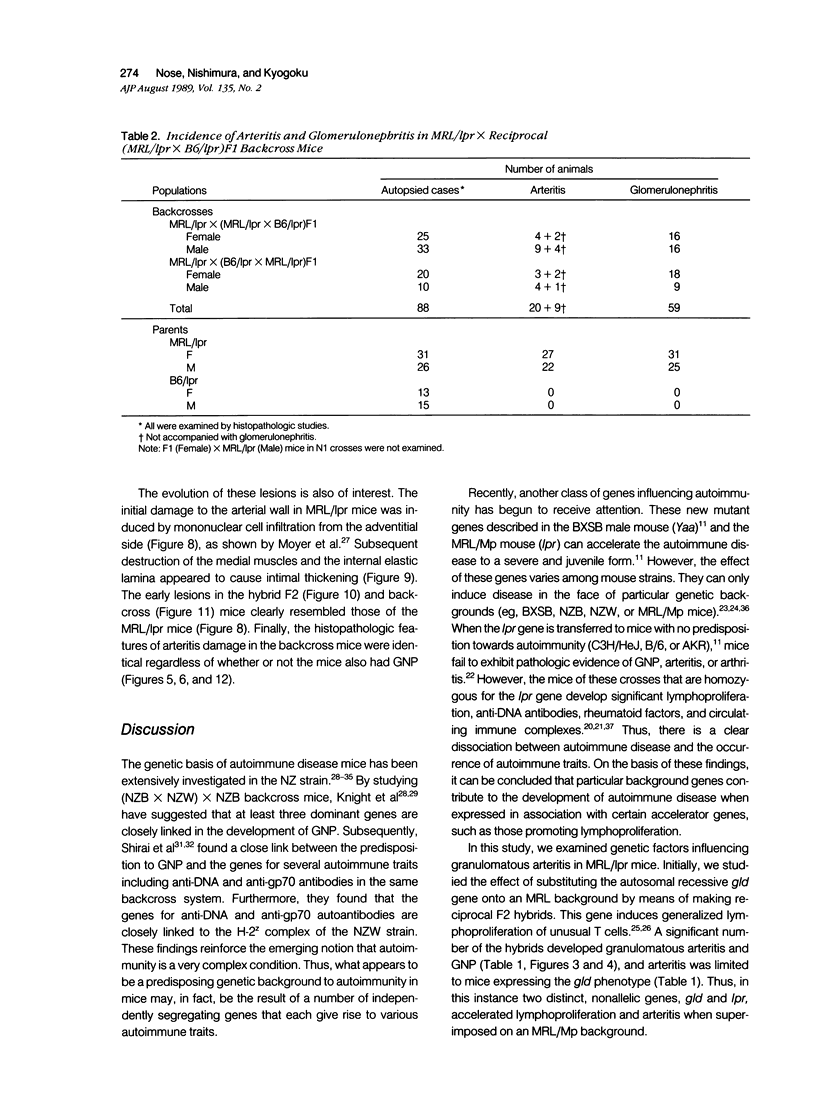
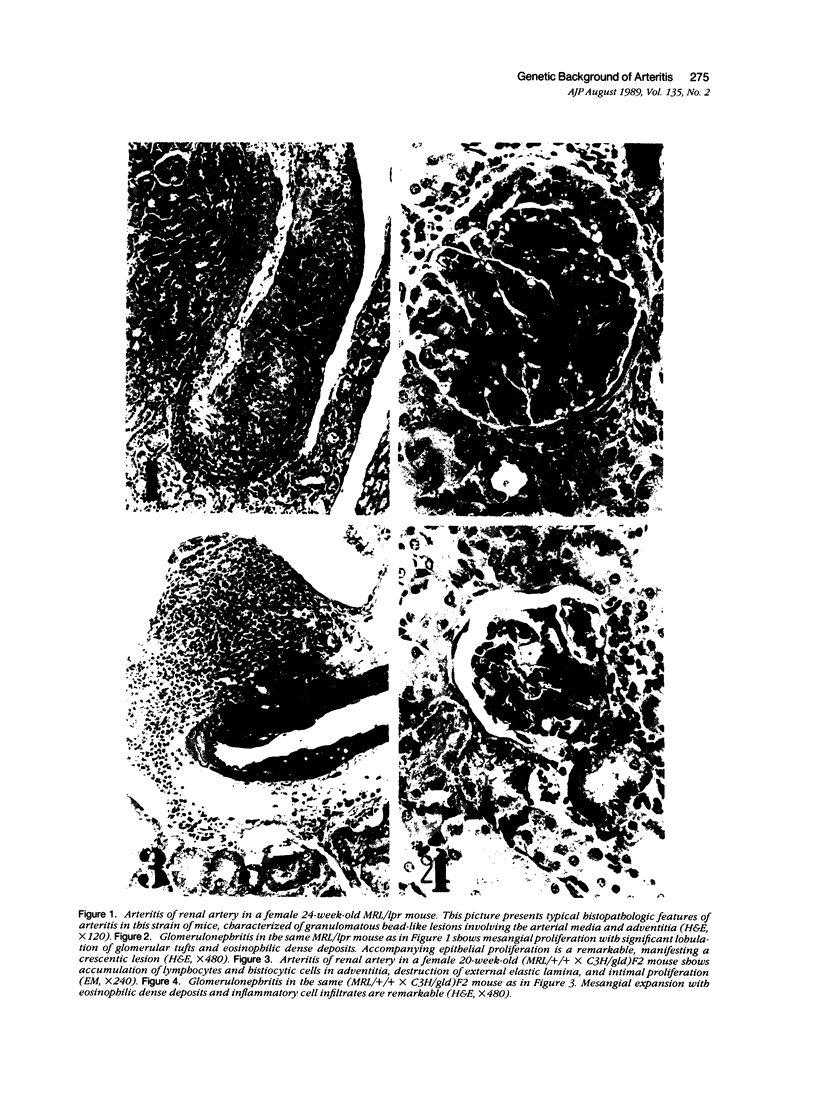
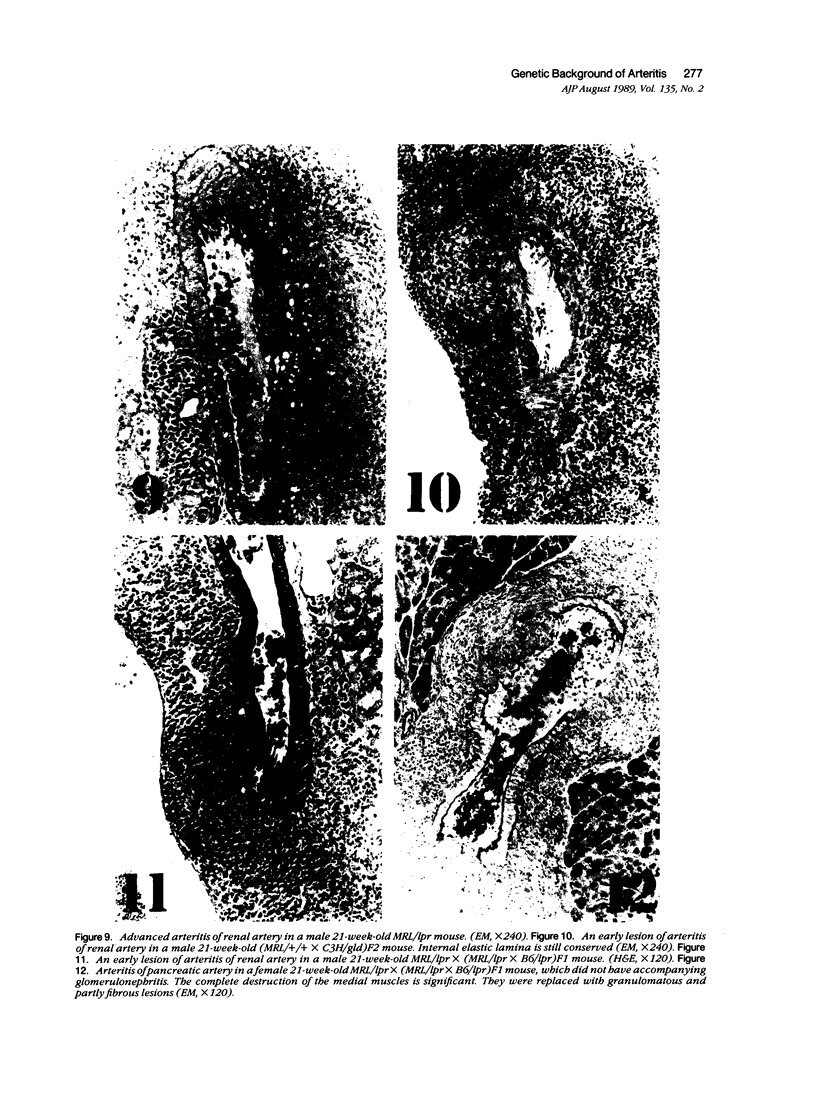
MRL/Mp mice bearing the lymphoproliferation gene (lpr) spontaneously develop systemic granulomatous arteritis coincident with glomerulonephritis (GNP). Although the association of lpr-dependent lymphoproliferation in these mice seems to be a prerequisite for the development of granulomatous arteritis, the genetic basis is poorly understood. The first approach to this problem was to study the ability of another, nonallelic, lymphoproliferative gene, gld (generalized lymphoproliferative disease), inducing arteritis in MRL/Mp mice. The gld gene was placed on an MRL/Mp background by producing reciprocal (MRL/Mp-+/+ X C3H/Hej-gld/gld)F2 hybrid mice. Seventeen percent of these mice with lymphoproliferation had arteritis and GNP, suggesting that more than one lymphoproliferative gene could induce GNP and arteritis in an MRL/Mp background. Next, the effect of rearrangements in the genetic background of MRL/Mp-lpr/lpr mice by hybridization with non-autoimmune lpr-bearing mice was examined. This was done by making MRL/Mp-lpr/lpr X reciprocal (MRL/Mp-lpr/lpr X C57BL/6-lpr/lpr)F1 mice. Thirty-three percent of these mice developed arteritis, but one third of these did not get GNP, thus showing that susceptibility to arteritis was separate from GNP. The histopathologic features of the arteritis in both the F2 hybrids and the backcross mice were granulomatous and were identical to those seen in MRL/Mp-lpr/lpr mice. These findings suggested that it might be possible to dissociated two components (arteritis and GNP) of a severe autoimmune disease of MRL/Mp mice and to study their pathogenesis separately.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accinni L., Dixon F. J. Degenerative vascular disease and myocardial infarction in mice with lupus-like syndrome. Am J Pathol. 1979 Aug;96(2):477–492. [PMC free article] [PubMed] [Google Scholar]
- Alexander E. L., Moyer C., Travlos G. S., Roths J. B., Murphy E. D. Two histopathologic types of inflammatory vascular disease in MRL/Mp autoimmune mice. Model for human vasculitis in connective tissue disease. Arthritis Rheum. 1985 Oct;28(10):1146–1155. doi: 10.1002/art.1780281011. [DOI] [PubMed] [Google Scholar]
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berden J. H., Hang L., McConahey P. J., Dixon F. J. Analysis of vascular lesions in murine SLE. I. Association with serologic abnormalities. J Immunol. 1983 Apr;130(4):1699–1705. [PubMed] [Google Scholar]
- Boswell J. M., Yui M. A., Endres S., Burt D. W., Kelley V. E. Novel and enhanced IL-1 gene expression in autoimmune mice with lupus. J Immunol. 1988 Jul 1;141(1):118–124. [PubMed] [Google Scholar]
- Christian C. L., Sergent J. S. Vasculitis syndromes: clinical and experimental models. Am J Med. 1976 Sep;61(3):385–392. doi: 10.1016/0002-9343(76)90376-4. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Koffler D. Immune complex disease in experimental animals and man. Adv Immunol. 1973;16(0):185–264. doi: 10.1016/s0065-2776(08)60298-9. [DOI] [PubMed] [Google Scholar]
- Davidson W. F., Holmes K. L., Roths J. B., Morse H. C., 3rd Immunologic abnormalities of mice bearing the gld mutation suggest a common pathway for murine nonmalignant lymphoproliferative disorders with autoimmunity. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1219–1223. doi: 10.1073/pnas.82.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson W. F., Roths J. B., Holmes K. L., Rudikoff E., Morse H. C., 3rd Dissociation of severe lupus-like disease from polyclonal B cell activation and IL 2 deficiency in C3H-lpr/lpr mice. J Immunol. 1984 Aug;133(2):1048–1056. [PubMed] [Google Scholar]
- Eastcott J. W., Schwartz R. S., Datta S. K. Genetic analysis of the inheritance of B cell hyperactivity in relation to the development of autoantibodies and glomerulonephritis in NZB x SWR crosses. J Immunol. 1983 Nov;131(5):2232–2239. [PubMed] [Google Scholar]
- Fauci A. S., Haynes B., Katz P. The spectrum of vasculitis: clinical, pathologic, immunologic and therapeutic considerations. Ann Intern Med. 1978 Nov;89(5 Pt 1):660–676. doi: 10.7326/0003-4819-89-5-660. [DOI] [PubMed] [Google Scholar]
- Gamble C. N., Wiesner K. B., Shapiro R. F., Boyer W. J. The immune complex pathogenesis of glomerulonephritis and pulmonary vasculitis in Behçet's disease. Am J Med. 1979 Jun;66(6):1031–1039. doi: 10.1016/0002-9343(79)90461-3. [DOI] [PubMed] [Google Scholar]
- Hicks J. D. Vascular changes in the kidneys of NZB mice and F1 NZBxNZW hybrids. J Pathol Bacteriol. 1966 Apr;91(2):479–486. doi: 10.1002/path.1700910222. [DOI] [PubMed] [Google Scholar]
- Hirose S., Nagasawa R., Sekikawa I., Hamaoki M., Ishida Y., Sato H., Shirai T. Enhancing effect of H-2-linked NZW gene(s) on the autoimmune traits of (NZB X NZW)F1 mice. J Exp Med. 1983 Jul 1;158(1):228–233. doi: 10.1084/jem.158.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose S., Ueda G., Noguchi K., Okada T., Sekigawa I., Sato H., Shirai T. Requirement of H-2 heterozygosity for autoimmunity in (NZB X NZW)F1 hybrid mice. Eur J Immunol. 1986 Dec;16(12):1631–1633. doi: 10.1002/eji.1830161226. [DOI] [PubMed] [Google Scholar]
- Izui S., Kelley V. E., Masuda K., Yoshida H., Roths J. B., Murphy E. D. Induction of various autoantibodies by mutant gene lpr in several strains of mice. J Immunol. 1984 Jul;133(1):227–233. [PubMed] [Google Scholar]
- Kelley V. E., Roths J. B. Increase in macrophage Ia expression in autoimmune mice: role of the Ipr gene. J Immunol. 1982 Sep;129(3):923–925. [PubMed] [Google Scholar]
- Knight J. G., Adams D. D., Purves H. D. The genetic contribution of the NZB mouse to the renal disease of the NZB x NZW hybrid. Clin Exp Immunol. 1977 May;28(2):352–358. [PMC free article] [PubMed] [Google Scholar]
- Knight J. G., Adams D. D. Three genes for lupus nephritis in NZB x NZW mice. J Exp Med. 1978 Jun 1;147(6):1653–1660. doi: 10.1084/jem.147.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler R., Schreiber R. D., Dixon F. J., Theofilopoulos A. N. Macrophage I-A/I-E expression and macrophage-stimulating lymphokines in murine lupus. Cell Immunol. 1984 Aug;87(1):92–100. doi: 10.1016/0008-8749(84)90133-3. [DOI] [PubMed] [Google Scholar]
- Kyogoku M., Kawashima M., Nose M., Yaginuma N., Nagao K. Pathological studies on the kidney of systemic arteritis. II. Animal model: comparative studies on the pathogenesis of arteritis and glomerulonephritis of SL/Ni and MRL/1. Nihon Jinzo Gakkai Shi. 1981 Jul;23(7):913–920. [PubMed] [Google Scholar]
- Maruyama N., Furukawa F., Nakai Y., Sasaki Y., Ohta K., Ozaki S., Hirose S., Shirai T. Genetic studies of autoimmunity in New Zealand mice. IV. Contribution of NZB and NZW genes to the spontaneous occurrence of retroviral gp70 immune complexes in (NZB X NZW)F1 hybrid and the correlation to renal disease. J Immunol. 1983 Feb;130(2):740–746. [PubMed] [Google Scholar]
- Miyazawa M., Nose M., Kawashima M., Kyogoku M. Pathogenesis of arteritis of SL/Ni mice. Possible lytic effect of anti-gp70 antibodies on vascular smooth muscle cells. J Exp Med. 1987 Oct 1;166(4):890–908. doi: 10.1084/jem.166.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Davidson W. F., Yetter R. A., Murphy E. D., Roths J. B., Coffman R. L. Abnormalities induced by the mutant gene Ipr: expansion of a unique lymphocyte subset. J Immunol. 1982 Dec;129(6):2612–2615. [PubMed] [Google Scholar]
- Moyer C. F., Strandberg J. D., Reinisch C. L. Systemic mononuclear-cell vasculitis in MRL/Mp-lpr/lpr mice. A histologic and immunocytochemical analysis. Am J Pathol. 1987 May;127(2):229–242. [PMC free article] [PubMed] [Google Scholar]
- Nemazee D. A., Studer S., Steinmetz M., Dembić Z., Kiefer M. The lymphoproliferating cells of MRL-lpr/lpr mice are a polyclonal population that bear the T lymphocyte receptor for antigen. Eur J Immunol. 1985 Aug;15(8):760–764. doi: 10.1002/eji.1830150804. [DOI] [PubMed] [Google Scholar]
- Palacios R. Spontaneous production of interleukin 3 by T lymphocytes from autoimmune MRL/MP-lpr/lpr mice. Eur J Immunol. 1984 Jul;14(7):599–605. doi: 10.1002/eji.1830140704. [DOI] [PubMed] [Google Scholar]
- Pearson C. M., Bohan A. The spectrum of polymyositis and dermatomyositis. Med Clin North Am. 1977 Mar;61(2):439–457. doi: 10.1016/s0025-7125(16)31343-8. [DOI] [PubMed] [Google Scholar]
- Prud'Homme G. J., Park C. L., Fieser T. M., Kofler R., Dixon F. J., Theofilopoulos A. N. Identification of a B cell differentiation factor(s) spontaneously produced by proliferating T cells in murine lupus strains of the lpr/lpr genotype. J Exp Med. 1983 Feb 1;157(2):730–742. doi: 10.1084/jem.157.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveché E. S., Steinberg A. D., Klassen L. W., Tjio J. H. Genetic studies in NZB mice. I. Spontaneous autoantibody production. J Exp Med. 1978 May 1;147(5):1487–1502. doi: 10.1084/jem.147.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roths J. B., Murphy E. D., Eicher E. M. A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp Med. 1984 Jan 1;159(1):1–20. doi: 10.1084/jem.159.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer P. A., McEvilly R. J., Noonan D. J., Dixon F. J., Theofilopoulos A. N. Clonal diversity and T-cell receptor beta-chain variable gene expression in enlarged lymph nodes of MRL-lpr/lpr lupus mice. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7018–7022. doi: 10.1073/pnas.83.18.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Wigley R. D., Couchman K. G. Polyarteritis nodosa-like disease in outbred mice. Nature. 1966 Jul 16;211(5046):319–320. doi: 10.1038/211319a0. [DOI] [PubMed] [Google Scholar]
- Wofsy D., Hardy R. R., Seaman W. E. The proliferating cells in autoimmune MRL/lpr mice lack L3T4, an antigen on "helper" T cells that is involved in the response to class II major histocompatibility antigens. J Immunol. 1984 Jun;132(6):2686–2689. [PubMed] [Google Scholar]
- Wofsy D., Murphy E. D., Roths J. B., Dauphinée M. J., Kipper S. B., Talal N. Deficient interleukin 2 activity in MRL/Mp and C57BL/6J mice bearing the lpr gene. J Exp Med. 1981 Nov 1;154(5):1671–1680. doi: 10.1084/jem.154.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Kohno A., Ohta K., Hirose S., Maruyama N., Shirai T. Genetic studies of autoimmunity in New Zealand mice. III. Associations among anti-DNA antibodies, NTA, and renal disease in (NZB x NZW)F1 x NZW backcross mice. J Immunol. 1981 Aug;127(2):433–437. [PubMed] [Google Scholar]