Abstract
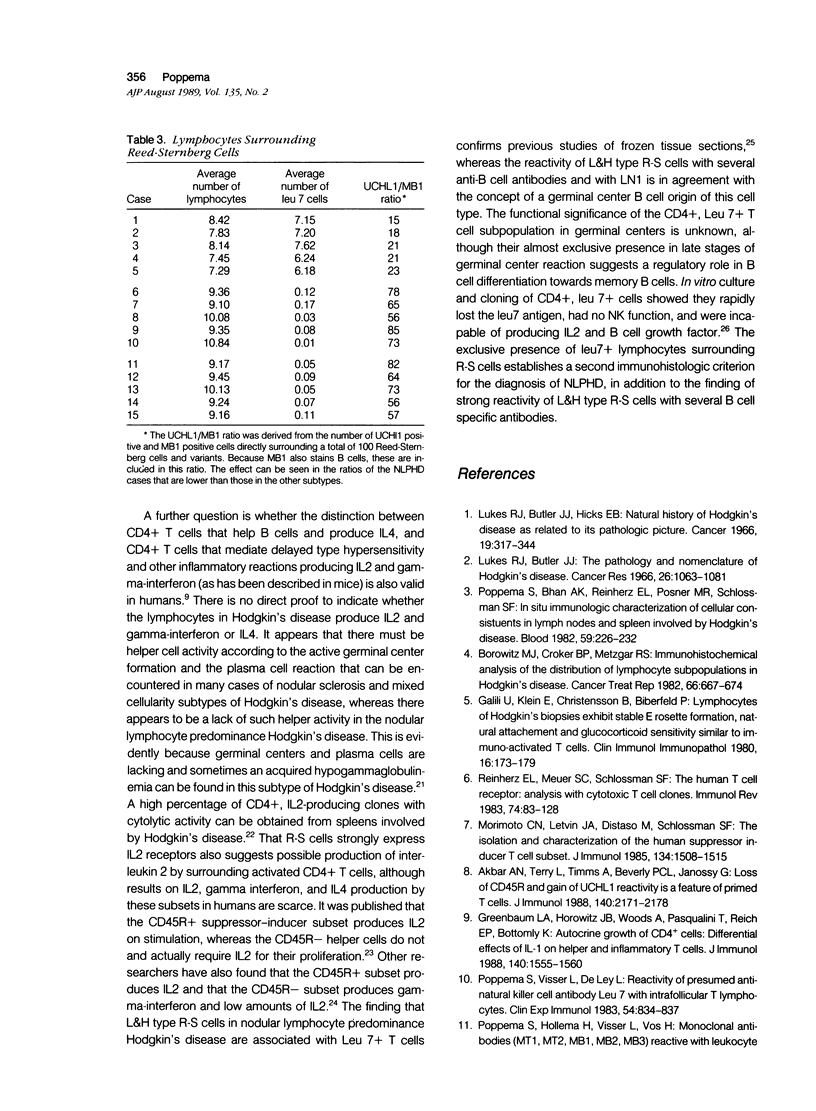
The lymphocytes surrounding Reed-Sternberg cells may play an important role in the pathogenesis of Hodgkin's disease. In this study, T cells in different subtypes of Hodgkin's disease were analyzed in situ by an immunoperoxidase method employing a panel of antibodies, including several paraffin tissue-reactive monoclonal antibodies. The T cells in Hodgkin's disease-involved tissues were found to be activated CD4-positive T cells that are UCHL1+ and CD45R-. This immunophenotype is compatible with an activated helper-inducer memory T cell population. The T cells in the nodular lymphocyte predominance subtype were found to have additional positivity for Leu 7, indicating a subpopulation of CD4+ T cells, normally confined to the light zone of germinal centers of secondary follicles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Beverley P. C., Merkenschlager M., Terry L. Phenotypic diversity of the CD45 antigen and its relationship to function. Immunol Suppl. 1988;1:3–5. [PubMed] [Google Scholar]
- Borowitz M. J., Croker B. P., Metzgar R. S. Immunohistochemical analysis of the distribution of lymphocyte subpopulations in Hodgkin's disease. Cancer Treat Rep. 1982 Apr;66(4):667–674. [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Dohlsten M., Hedlund G., Sjögren H. O., Carlsson R. Two subsets of human CD4+ T helper cells differing in kinetics and capacities to produce interleukin 2 and interferon-gamma can be defined by the Leu-18 and UCHL1 monoclonal antibodies. Eur J Immunol. 1988 Aug;18(8):1173–1178. doi: 10.1002/eji.1830180805. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Sanders M. E., Shaw S., Springer T. A. Purified lymphocyte function-associated antigen 3 binds to CD2 and mediates T lymphocyte adhesion. J Exp Med. 1987 Mar 1;165(3):677–692. doi: 10.1084/jem.165.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein A. L., Marder R. J., Winter J. N., Fox R. I. Two new monoclonal antibodies (LN-1, LN-2) reactive in B5 formalin-fixed, paraffin-embedded tissues with follicular center and mantle zone human B lymphocytes and derived tumors. J Immunol. 1984 Aug;133(2):1028–1036. [PubMed] [Google Scholar]
- Galili U., Klein E., Christensson B., Biberfeld P. Lymphocytes of Hodgkin's biopsies exhibit: stable E-rosette formation, natural attachment and glucocorticoid sensitivity, similar to immunoactivated T cells. Clin Immunol Immunopathol. 1980 Jun;16(2):173–179. doi: 10.1016/0090-1229(80)90201-9. [DOI] [PubMed] [Google Scholar]
- Greenbaum L. A., Horowitz J. B., Woods A., Pasqualini T., Reich E. P., Bottomly K. Autocrine growth of CD4+ T cells. Differential effects of IL-1 on helper and inflammatory T cells. J Immunol. 1988 Mar 1;140(5):1555–1560. [PubMed] [Google Scholar]
- Lukes R. J., Butler J. J. The pathology and nomenclature of Hodgkin's disease. Cancer Res. 1966 Jun;26(6):1063–1083. [PubMed] [Google Scholar]
- Marder R. J., Variakojis D., Silver J., Epstein A. L. Immunohistochemical analysis of human lymphomas with monoclonal antibodies to B cell and Ia antigens reactive in paraffin sections. Lab Invest. 1985 May;52(5):497–504. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Poppema S., Bhan A. K., Reinherz E. L., Posner M. R., Schlossman S. F. In situ immunologic characterization of cellular constituents in lymph nodes and spleens involved by Hodgkin's disease. Blood. 1982 Feb;59(2):226–232. [PubMed] [Google Scholar]
- Poppema S., Visser L., De Leij L. Reactivity of presumed anti-natural killer cell antibody Leu 7 with intrafollicular T lymphocytes. Clin Exp Immunol. 1983 Dec;54(3):834–837. [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S. C., Schlossman S. F. The human T cell receptor: analysis with cytotoxic T cell clones. Immunol Rev. 1983;74:83–112. doi: 10.1111/j.1600-065x.1983.tb01085.x. [DOI] [PubMed] [Google Scholar]
- Romagnani S., Maggi E., Parronchi P., Macchia D., Del Prete G. F., Rossi-Ferrini P. L., Ricci M., Moretta L. Clonal analysis of T lymphocytes in spleens from patients with Hodgkin's disease. Frequent occurrence of unusual T4-positive cells which co-express cytolytic activity and production of interleukin-2. Int J Cancer. 1986 Mar 15;37(3):343–349. doi: 10.1002/ijc.2910370304. [DOI] [PubMed] [Google Scholar]
- Salmon M., Kitas G. D., Gaston J. S., Bacon P. A. Interleukin-2 production and response by helper T-cell subsets in man. Immunology. 1988 Sep;65(1):81–85. [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sussman E. H., Luce G. E., Cossman J., Shaw S. Molecular pathways of adhesion in spontaneous rosetting of T-lymphocytes to the Hodgkin's cell line L428. Cancer Res. 1988 Jan 1;48(1):37–40. [PubMed] [Google Scholar]
- Serra H. M., Krowka J. F., Ledbetter J. A., Pilarski L. M. Loss of CD45R (Lp220) represents a post-thymic T cell differentiation event. J Immunol. 1988 Mar 1;140(5):1435–1441. [PubMed] [Google Scholar]
- Timens W., Visser L., Poppema S. Nodular lymphocyte predominance type of Hodgkin's disease is a germinal center lymphoma. Lab Invest. 1986 Apr;54(4):457–461. [PubMed] [Google Scholar]
- Velardi A., Mingari M. C., Moretta L., Grossi C. E. Functional analysis of cloned germinal center CD4+ cells with natural killer cell-related features. Divergence from typical T helper cells. J Immunol. 1986 Nov 1;137(9):2808–2813. [PubMed] [Google Scholar]




