Abstract
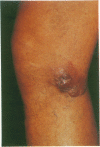
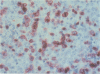
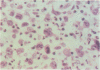
Skin biopsies were collected from 15 patients with cutaneous tumors morphologically similar to Ki-1+ anaplastic large cell (ALC) lymphoma of the lymph nodes, including Ki-1+ ALC lymphoma of childhood. The histology and immunophenotype of these cutaneous tumors are reported and follow-up data on the patients are given. The tumorous infiltrates were composed of large, sometimes very bizarre cells with one nucleolus or multiple nucleoli. All tumor cells expressed the lymphoid cell activation antigen Ki-1 (CD30) in conjunction with CD25 and the beta-chain of the T cell receptor. In 11 patients, Ki-1+ cutaneous tumors developed primarily in the skin. In nine patients, these were restricted to the skin in follow-up periods ranging from 3 months to 6 years. Two patients developed lymph node involvement after 2 months and 2 years, indicating the spreading potential of these cutaneous tumors. Morphologic and immunophenotypical identity of the atypical cells found in primary cutaneous ALC lymphoma, in regressing atypical histiocytosis (RAH), and in lymphomatoid papulosis (LyP) of type A, together with the protracted clinical course in all three conditions, suggests that primary cutaneous ALC lymphoma, RAH, and LyP of type A represent clinical variants of the same lymphoma entity. Secondary development of Ki-1+ ALC skin tumors was observed in two patients with cutaneous T cell lymphomas of mycosis fungoides type. These secondary Ki-1+ ALC lymphomas of the skin showed rapid systemic progression similar to the primary lymphonodal Ki-1+ ALC lymphomas. Concomitant or subsequent occurrence of Ki-1+ ALC tumors in cutaneous T cell lymphomas thus may be a bad prognostic sign.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnarsson B. A., Kadin M. E. Ki-1 positive large cell lymphoma. A morphologic and immunologic study of 19 cases. Am J Surg Pathol. 1988 Apr;12(4):264–274. doi: 10.1097/00000478-198804000-00002. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Scheft H., Warnke R. A., Jones N., Strominger J. L. Characterization and expression of the human alpha beta T cell receptor by using a framework monoclonal antibody. J Immunol. 1987 Mar 1;138(5):1502–1509. [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Flynn K. J., Dehner L. P., Gajl-Peczalska K. J., Dahl M. V., Ramsay N., Wang N. Regressing atypical histiocytosis: a cutaneous proliferation of atypical neoplastic histiocytes with unexpectedly indolent biologic behavior. Cancer. 1982 Mar 1;49(5):959–970. doi: 10.1002/1097-0142(19820301)49:5<959::aid-cncr2820490521>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983 Jan 15;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Headington J. T., Roth M. S., Ginsburg D., Lichter A. S., Hyder D., Schnitzer B. T-cell receptor gene rearrangement in regressing atypical histiocytosis. Arch Dermatol. 1987 Sep;123(9):1183–1187. [PubMed] [Google Scholar]
- Kadin M. E., Sako D., Berliner N., Franklin W., Woda B., Borowitz M., Ireland K., Schweid A., Herzog P., Lange B. Childhood Ki-1 lymphoma presenting with skin lesions and peripheral lymphadenopathy. Blood. 1986 Nov;68(5):1042–1049. [PubMed] [Google Scholar]
- Kadin M., Nasu K., Sako D., Said J., Vonderheid E. Lymphomatoid papulosis. A cutaneous proliferation of activated helper T cells expressing Hodgkin's disease-associated antigens. Am J Pathol. 1985 May;119(2):315–325. [PMC free article] [PubMed] [Google Scholar]
- Kaudewitz P., Stein H., Burg G., Mason D. Y., Braun-Falco O. Atypical cells in lymphomatoid papulosis express the Hodgkin cell-associated antigen Ki-1. J Invest Dermatol. 1986 Apr;86(4):350–354. doi: 10.1111/1523-1747.ep12285562. [DOI] [PubMed] [Google Scholar]
- Macaulay W. L. Lymphomatoid papulosis. A continuing self-healing eruption, clinically benign--histologically malignant. Arch Dermatol. 1968 Jan;97(1):23–30. doi: 10.1001/archderm.97.1.23. [DOI] [PubMed] [Google Scholar]
- Ralfkiaer E., Bosq J., Gatter K. C., Schwarting R., Gerdes J., Stein H., Mason D. Y. Expression of a Hodgkin and Reed-Sternberg cell associated antigen (Ki-1) in cutaneous lymphoid infiltrates. Arch Dermatol Res. 1987;279(5):285–292. doi: 10.1007/BF00431219. [DOI] [PubMed] [Google Scholar]
- Salhany K. E., Cousar J. B., Greer J. P., Casey T. T., Fields J. P., Collins R. D. Transformation of cutaneous T cell lymphoma to large cell lymphoma. A clinicopathologic and immunologic study. Am J Pathol. 1988 Aug;132(2):265–277. [PMC free article] [PubMed] [Google Scholar]
- Sanchez N. P., Pittelkow M. R., Muller S. A., Banks P. M., Winkelmann R. K. The clinicopathologic spectrum of lymphomatoid papulosis: study of 31 cases. J Am Acad Dermatol. 1983 Jan;8(1):81–94. doi: 10.1016/s0190-9622(83)70011-3. [DOI] [PubMed] [Google Scholar]
- Schwab U., Stein H., Gerdes J., Lemke H., Kirchner H., Schaadt M., Diehl V. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature. 1982 Sep 2;299(5878):65–67. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- Stansfeld A. G., Diebold J., Noel H., Kapanci Y., Rilke F., Kelényi G., Sundstrom C., Lennert K., van Unnik J. A., Mioduszewska O. Updated Kiel classification for lymphomas. Lancet. 1988 Feb 6;1(8580):292–293. doi: 10.1016/s0140-6736(88)90367-4. [DOI] [PubMed] [Google Scholar]
- Stein H., Gerdes J., Schwab U., Lemke H., Mason D. Y., Ziegler A., Schienle W., Diehl V. Identification of Hodgkin and Sternberg-reed cells as a unique cell type derived from a newly-detected small-cell population. Int J Cancer. 1982 Oct 15;30(4):445–459. doi: 10.1002/ijc.2910300411. [DOI] [PubMed] [Google Scholar]
- Stein H., Mason D. Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G., Gatter K., Falini B., Delsol G., Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985 Oct;66(4):848–858. [PubMed] [Google Scholar]
- Willemze R., Ruiter D. J., Van Vloten W. A., Meijer C. J. Reticulum cell sarcomas (large cell lymphomas) presenting in the skin. High frequency of true histiocytic lymphoma. Cancer. 1982 Oct 1;50(7):1367–1379. doi: 10.1002/1097-0142(19821001)50:7<1367::aid-cncr2820500724>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Ziegler A., Uchańska-Ziegler B., Zeuthen J., Wernet P. HLA antigen expression at the single cell level on a K562 X B cell hybrid: an analysis with monoclonal antibodies using bacterial binding assays. Somatic Cell Genet. 1982 Nov;8(6):775–789. doi: 10.1007/BF01543018. [DOI] [PubMed] [Google Scholar]