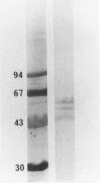
Abstract
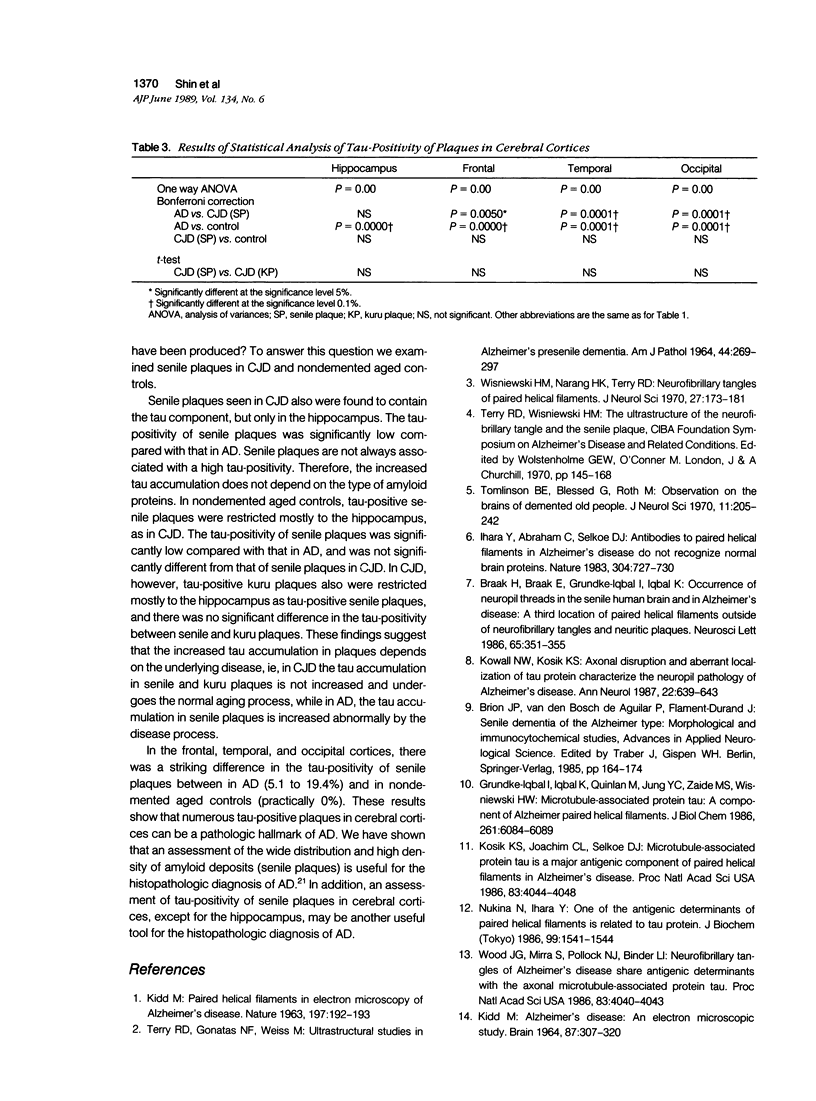
To identify the tau component in senile or kuru plaques, the authors examined brain sections from 12 patients with Alzheimer's disease (AD), 6 with Creutzfeldt-Jakob disease (CJD), and 20 nondemented aged controls using anti-beta protein, anti-buman prion protein, and affinity-purified tau-specific antibody. The tau component was identified both in senile and kuru plaques. In AD, tau-positive senile plaques were found in all cerebral cortices of almost all cases, and the tau-positivity of plaques in cerebral cortices was 5.1 to 27.5%. In CJD, tau-positive senile and kuru plaques were restricted to the hippocampus, and the tau-positivity was 4.3 and 1.2%, respectively. In nondemented aged controls, tau-positive senile plaques also were restricted mostly to the hippocampus, and the tau-positivity was 1.3%. Significant differences in the tau-positivity of senile plaques were found between AD and CJD and nondemented aged controls, and no significant differences were found between CJD and nondemented aged controls. These observations are important because increased tau accumulation in senile plaques can be a hallmark of AD.
Full text
PDF

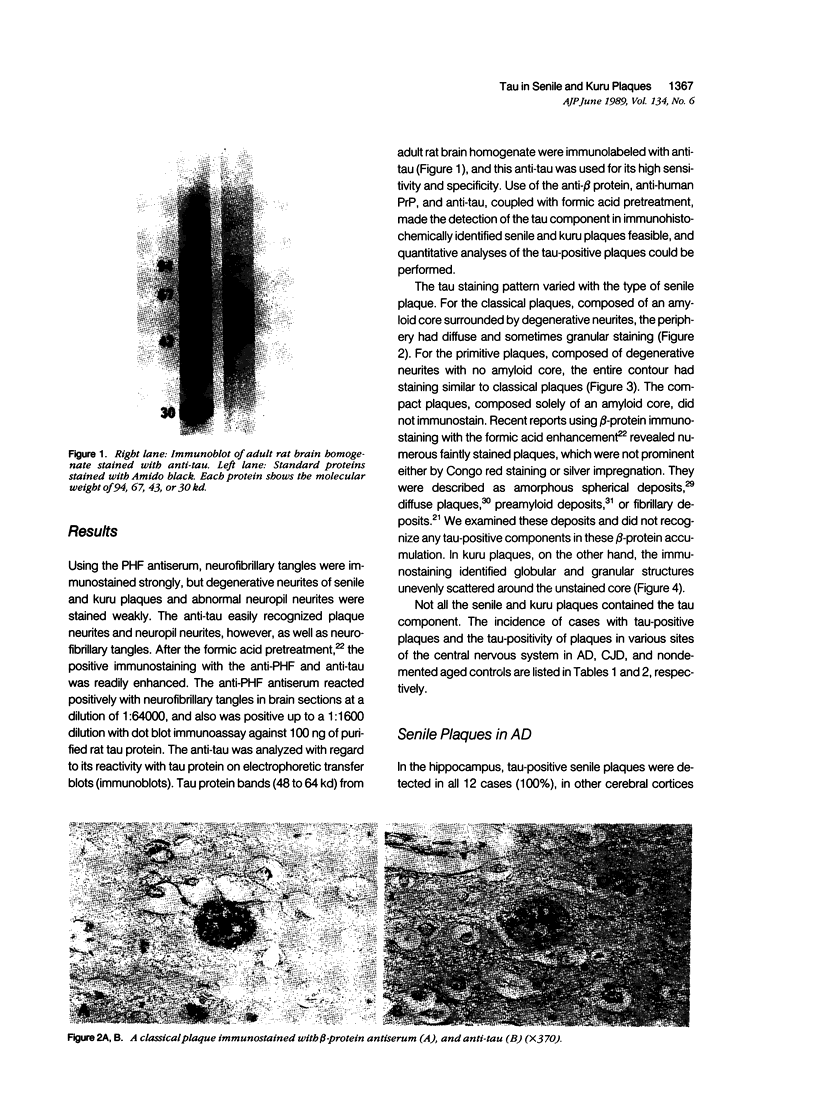
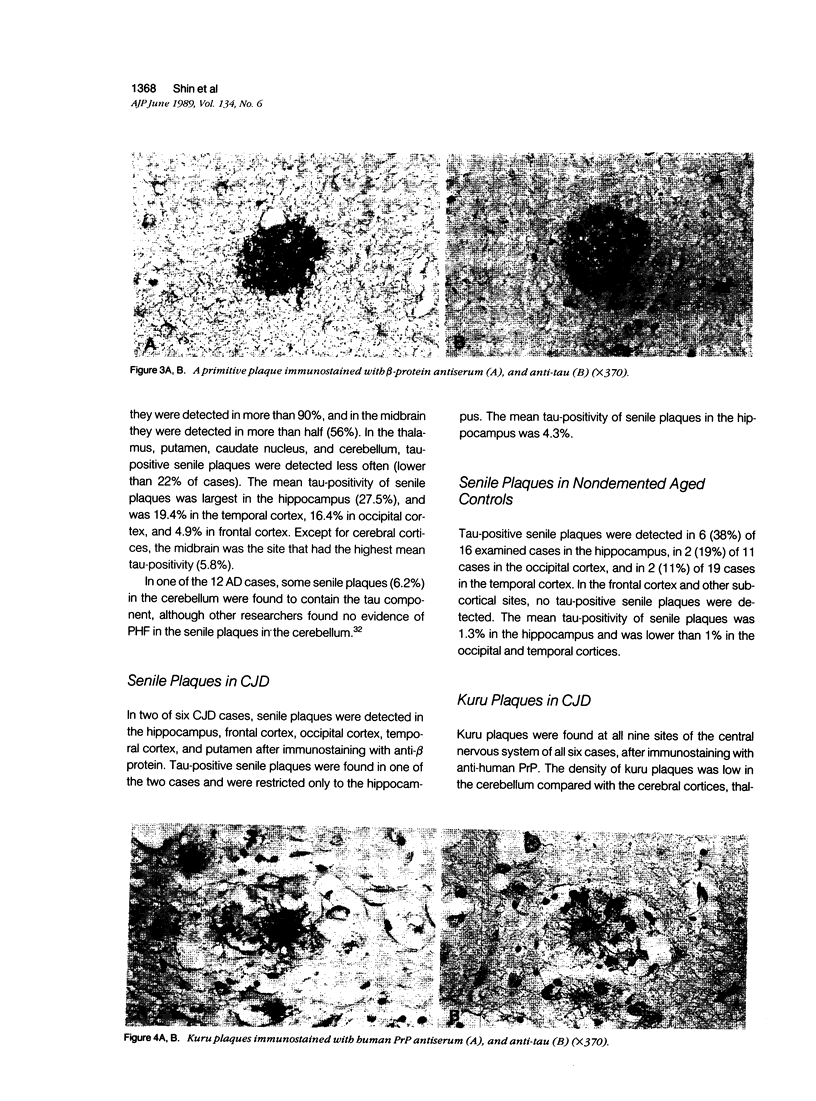
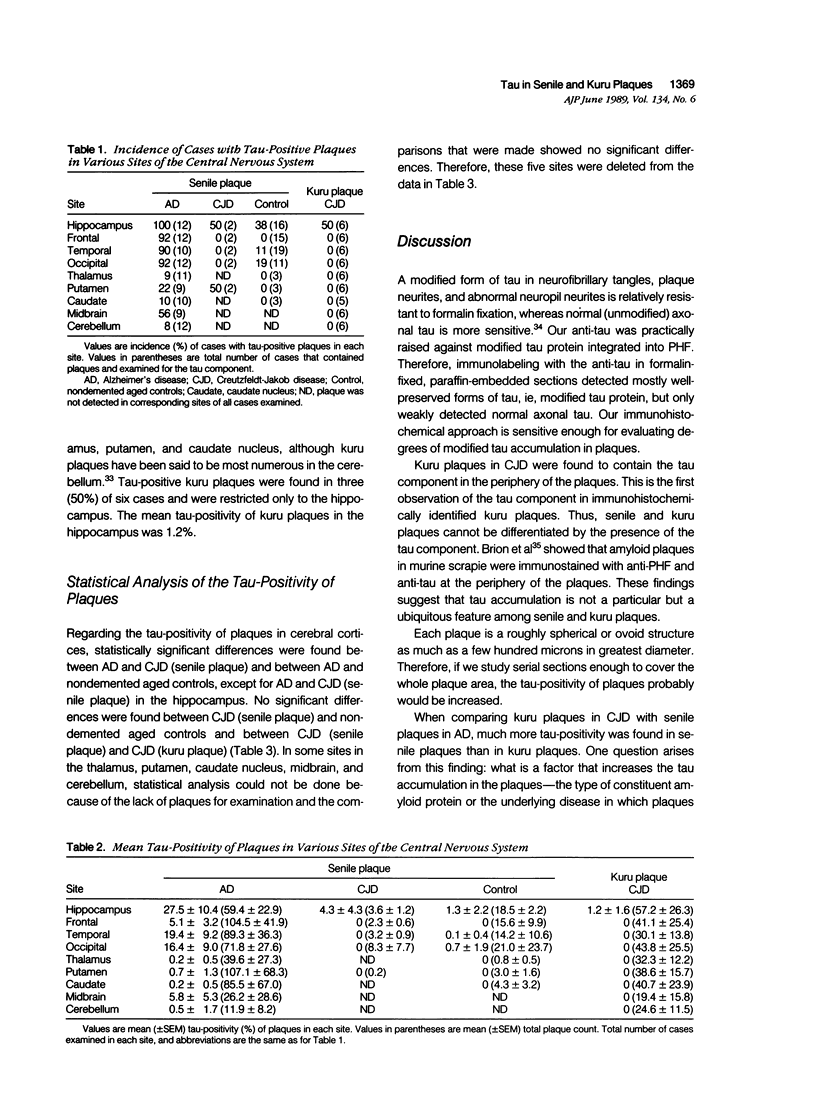

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braak H., Braak E., Grundke-Iqbal I., Iqbal K. Occurrence of neuropil threads in the senile human brain and in Alzheimer's disease: a third location of paired helical filaments outside of neurofibrillary tangles and neuritic plaques. Neurosci Lett. 1986 Apr 24;65(3):351–355. doi: 10.1016/0304-3940(86)90288-0. [DOI] [PubMed] [Google Scholar]
- Brion J. P., Fraser H., Flament-Durand J., Dickinson A. G. Amyloid scrapie plaques in mice, and Alzheimer senile plaques, share common antigens with tau, a microtubule-associated protein. Neurosci Lett. 1987 Jul 9;78(1):113–118. doi: 10.1016/0304-3940(87)90571-4. [DOI] [PubMed] [Google Scholar]
- Davies L., Wolska B., Hilbich C., Multhaup G., Martins R., Simms G., Beyreuther K., Masters C. L. A4 amyloid protein deposition and the diagnosis of Alzheimer's disease: prevalence in aged brains determined by immunocytochemistry compared with conventional neuropathologic techniques. Neurology. 1988 Nov;38(11):1688–1693. doi: 10.1212/wnl.38.11.1688. [DOI] [PubMed] [Google Scholar]
- Gorevic P. D., Goñi F., Pons-Estel B., Alvarez F., Peress N. S., Frangione B. Isolation and partial characterization of neurofibrillary tangles and amyloid plaque core in Alzheimer's disease: immunohistological studies. J Neuropathol Exp Neurol. 1986 Nov;45(6):647–664. doi: 10.1097/00005072-198611000-00004. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y. C., Zaidi M. S., Wisniewski H. M. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986 May 5;261(13):6084–6089. [PubMed] [Google Scholar]
- Hikita K., Tateishi J., Nagara H. Morphogenesis of amyloid plaques in mice with Creutzfeldt-Jakob disease. Acta Neuropathol. 1985;68(2):138–144. doi: 10.1007/BF00688635. [DOI] [PubMed] [Google Scholar]
- Ihara Y., Abraham C., Selkoe D. J. Antibodies to paired helical filaments in Alzheimer's disease do not recognize normal brain proteins. Nature. 1983 Aug 25;304(5928):727–730. doi: 10.1038/304727a0. [DOI] [PubMed] [Google Scholar]
- Ishino H., Higashi S., Chûta M., Ohta H. Juvenile Alzheimer's disease with myoclonus: amyloid plaques and grumose alteration in the cerebellum. Clin Neuropathol. 1984 Sep-Oct;3(5):193–198. [PubMed] [Google Scholar]
- Joachim C. L., Morris J. H., Kosik K. S., Selkoe D. J. Tau antisera recognize neurofibrillary tangles in a range of neurodegenerative disorders. Ann Neurol. 1987 Oct;22(4):514–520. doi: 10.1002/ana.410220411. [DOI] [PubMed] [Google Scholar]
- KIDD M. ALZHEIMER'S DISEASE--AN ELECTRON MICROSCOPICAL STUDY. Brain. 1964 Jun;87:307–320. doi: 10.1093/brain/87.2.307. [DOI] [PubMed] [Google Scholar]
- KIDD M. Paired helical filaments in electron microscopy of Alzheimer's disease. Nature. 1963 Jan 12;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Ogomori K., Tateishi J., Prusiner S. B. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest. 1987 Aug;57(2):230–236. [PubMed] [Google Scholar]
- Kitamoto T., Tateishi J. Immunohistochemical confirmation of Creutzfeldt-Jakob disease with a long clinical course with amyloid plaque core antibodies. Am J Pathol. 1988 Jun;131(3):435–443. [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T., Tateishi J., Sato Y. Immunohistochemical verification of senile and kuru plaques in Creutzfeldt-Jakob disease and the allied disease. Ann Neurol. 1988 Oct;24(4):537–542. doi: 10.1002/ana.410240410. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Joachim C. L., Selkoe D. J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowall N. W., Kosik K. S. Axonal disruption and aberrant localization of tau protein characterize the neuropil pathology of Alzheimer's disease. Ann Neurol. 1987 Nov;22(5):639–643. doi: 10.1002/ana.410220514. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nukina N., Ihara Y. One of the antigenic determinants of paired helical filaments is related to tau protein. J Biochem. 1986 May;99(5):1541–1544. doi: 10.1093/oxfordjournals.jbchem.a135625. [DOI] [PubMed] [Google Scholar]
- Ogomori K., Kitamoto T., Tateishi J., Sato Y., Suetsugu M., Abe M. Beta-protein amyloid is widely distributed in the central nervous system of patients with Alzheimer's disease. Am J Pathol. 1989 Feb;134(2):243–251. [PMC free article] [PubMed] [Google Scholar]
- Pollock N. J., Wood J. G. Differential sensitivity of the microtubule-associated protein, tau, in Alzheimer's disease tissue to formalin fixation. J Histochem Cytochem. 1988 Sep;36(9):1117–1121. doi: 10.1177/36.9.2841371. [DOI] [PubMed] [Google Scholar]
- Pro J. D., Smith C. H., Sumi S. M. Presenile Alzheimer disease: amyloid plaques in the cerebellum. Neurology. 1980 Aug;30(8):820–825. doi: 10.1212/wnl.30.8.820. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERRY R. D., GONATAS N. K., WEISS M. ULTRASTRUCTURAL STUDIES IN ALZHEIMER'S PRESENILE DEMENTIA. Am J Pathol. 1964 Feb;44:269–297. [PMC free article] [PubMed] [Google Scholar]
- Tagliavini F., Giaccone G., Frangione B., Bugiani O. Preamyloid deposits in the cerebral cortex of patients with Alzheimer's disease and nondemented individuals. Neurosci Lett. 1988 Nov 11;93(2-3):191–196. doi: 10.1016/0304-3940(88)90080-8. [DOI] [PubMed] [Google Scholar]
- Talian J. C., Olmsted J. B., Goldman R. D. A rapid procedure for preparing fluorescein-labeled specific antibodies from whole antiserum: its use in analyzing cytoskeletal architecture. J Cell Biol. 1983 Oct;97(4):1277–1282. doi: 10.1083/jcb.97.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson B. E., Blessed G., Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970 Sep;11(3):205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakili S. T., Muller J. Juvenile Alzheimer's disease with cerebellar involvement. Arch Pathol Lab Med. 1987 May;111(5):480–482. [PubMed] [Google Scholar]
- Wiśniewski H. M., Narang H. K., Terry R. D. Neurofibrillary tangles of paired helical filaments. J Neurol Sci. 1976 Feb;27(2):173–181. doi: 10.1016/0022-510x(76)90059-9. [DOI] [PubMed] [Google Scholar]
- Wood J. G., Mirra S. S., Pollock N. J., Binder L. I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986 Jun;83(11):4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Hirai S., Morimatsu M., Shoji M., Ihara Y. A variety of cerebral amyloid deposits in the brains of the Alzheimer-type dementia demonstrated by beta protein immunostaining. Acta Neuropathol. 1988;76(6):541–549. doi: 10.1007/BF00689591. [DOI] [PubMed] [Google Scholar]