Abstract
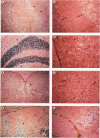
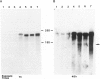
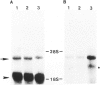
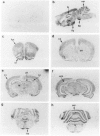
The neuritic plaque is a characteristic finding in Alzheimer's disease. A major component of the plaque core is a 4.2 kd polypeptide, amyloid beta-protein (ABP), which is derived from the C-terminus of a larger precursor protein (ABPP). The authors have studied the transcription of ABPP mRNA in the adult rat brain by Northern analysis and in situ hybridization, and report that the ABPP gene gives rise to essentially the same multitranscript family of mRNAs as in the human, and that differential transcription patterns exist between brain and kidney. Morphologically, ABPP mRNA is expressed ubiquitously in neurons of the fore and hindbrain. ABPP transcripts also are present less frequently in occasional glial cells and at moderate to low frequency in nonneural cell types, namely, the choroid plexus epithelium, ependymal cells, and leptomeningeal membranes. Neuronal transcripts are most abundant in cerebral cortical layers II and V, the pyramidal cell layer of the hippocampus, the olfactory cortex, nucleus basis pontis, cranial nerve nuclei, and, significantly, in Purkinje cells and cerebellar granule cells. Because the cerebellum is relatively uninvolved in Alzheimer's disease, these findings suggest that high intraneuronal expression of ABPP may be a necessary but not sufficient requirement for plaque formation.
Full text
PDF

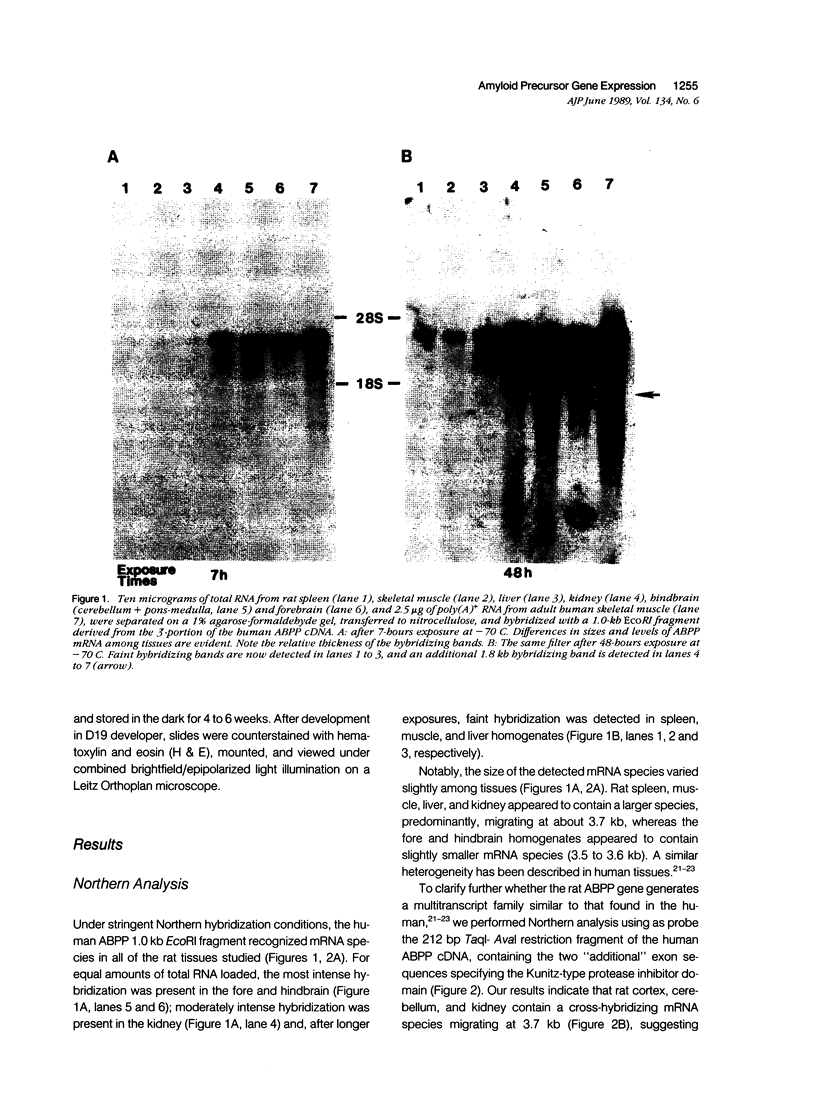
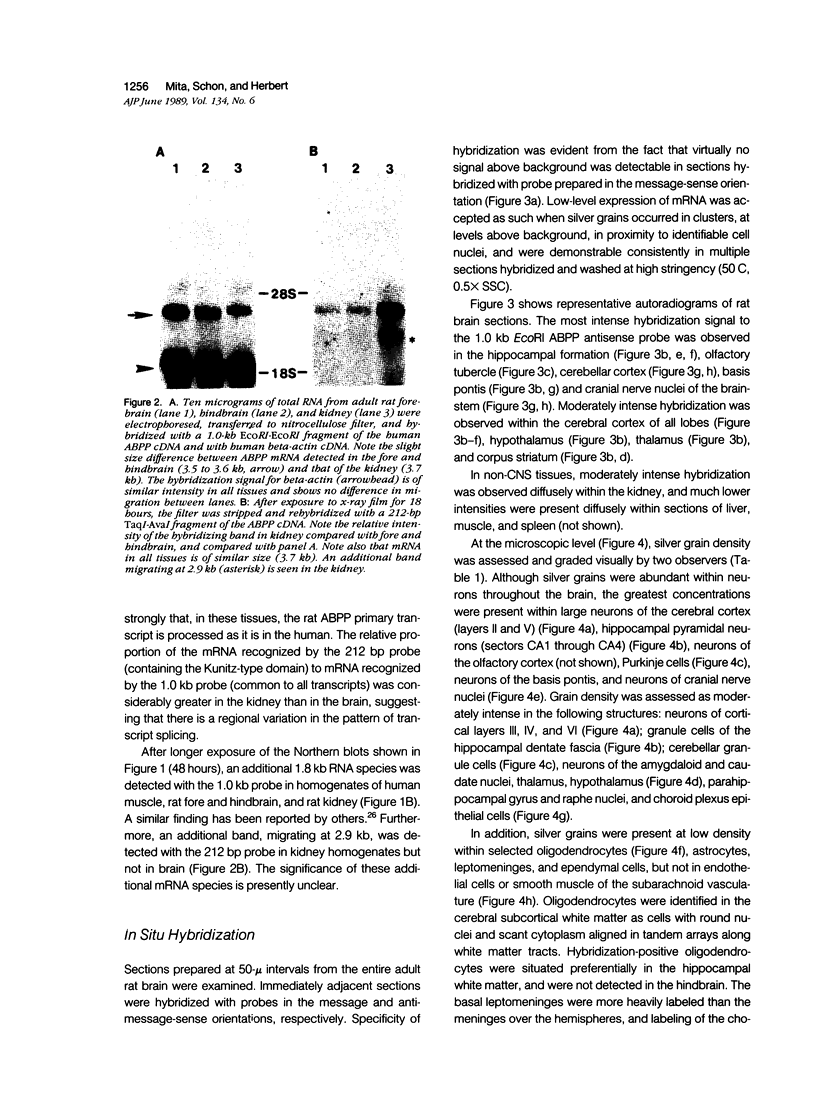
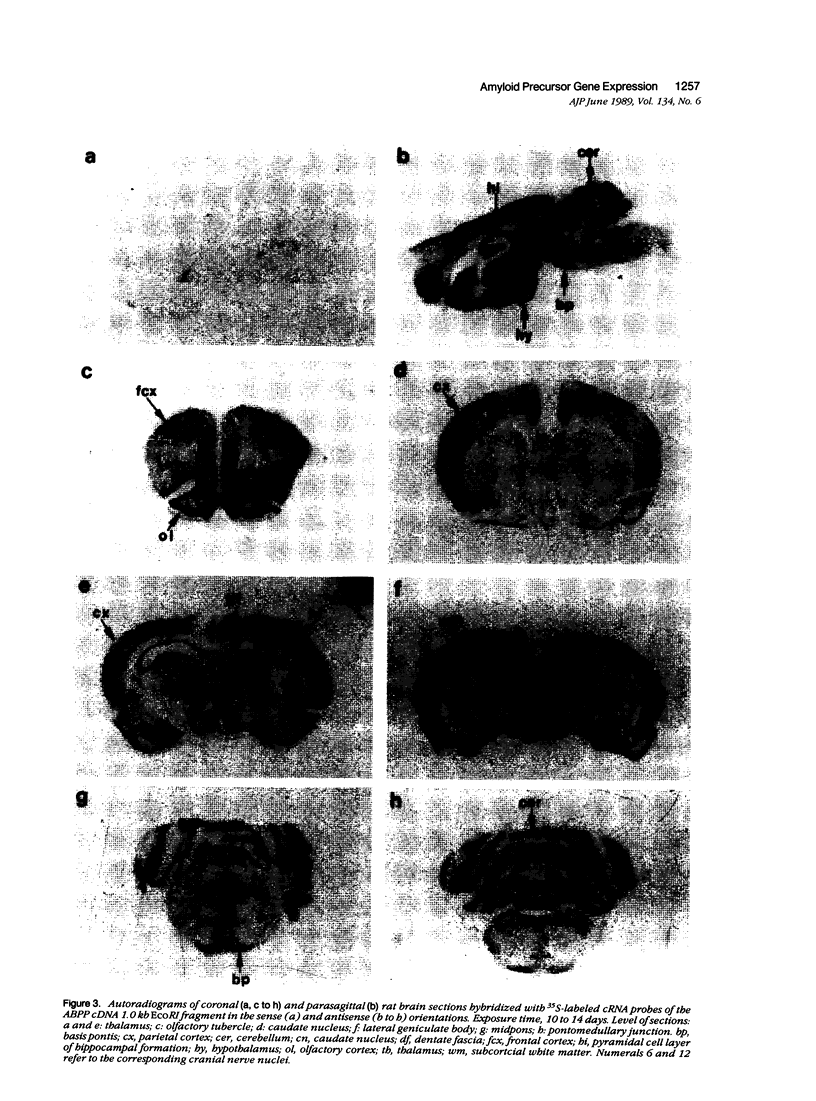
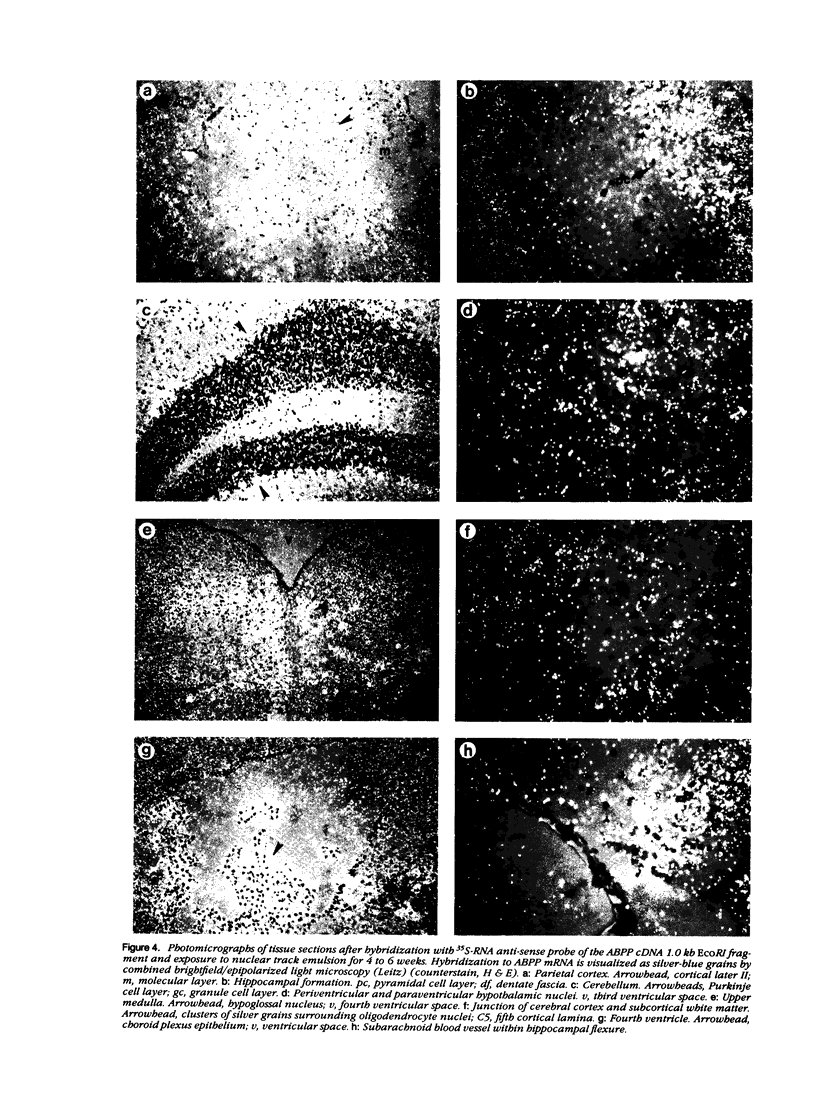



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham C. R., Selkoe D. J., Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988 Feb 26;52(4):487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- Allsop D., Wong C. W., Ikeda S., Landon M., Kidd M., Glenner G. G. Immunohistochemical evidence for the derivation of a peptide ligand from the amyloid beta-protein precursor of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2790–2794. doi: 10.1073/pnas.85.8.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahmanyar S., Higgins G. A., Goldgaber D., Lewis D. A., Morrison J. H., Wilson M. C., Shankar S. K., Gajdusek D. C. Localization of amyloid beta protein messenger RNA in brains from patients with Alzheimer's disease. Science. 1987 Jul 3;237(4810):77–80. doi: 10.1126/science.3299701. [DOI] [PubMed] [Google Scholar]
- Bendotti C., Forloni G. L., Morgan R. A., O'Hara B. F., Oster-Granite M. L., Reeves R. H., Gearhart J. D., Coyle J. T. Neuroanatomical localization and quantification of amyloid precursor protein mRNA by in situ hybridization in the brains of normal, aneuploid, and lesioned mice. Proc Natl Acad Sci U S A. 1988 May;85(10):3628–3632. doi: 10.1073/pnas.85.10.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. L., Golde T. E., Usiak M. F., Younkin L. H., Younkin S. G. In situ hybridization of nucleus basalis neurons shows increased beta-amyloid mRNA in Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1227–1231. doi: 10.1073/pnas.85.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delabar J. M., Goldgaber D., Lamour Y., Nicole A., Huret J. L., de Grouchy J., Brown P., Gajdusek D. C., Sinet P. M. Beta amyloid gene duplication in Alzheimer's disease and karyotypically normal Down syndrome. Science. 1987 Mar 13;235(4794):1390–1392. doi: 10.1126/science.2950593. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fremeau R. T., Jr, Lundblad J. R., Pritchett D. B., Wilcox J. N., Roberts J. L. Regulation of pro-opiomelanocortin gene transcription in individual cell nuclei. Science. 1986 Dec 5;234(4781):1265–1269. doi: 10.1126/science.3775385. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goedert M. Neuronal localization of amyloid beta protein precursor mRNA in normal human brain and in Alzheimer's disease. EMBO J. 1987 Dec 1;6(12):3627–3632. doi: 10.1002/j.1460-2075.1987.tb02694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., Ito H. Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature. 1988 Feb 11;331(6156):530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lewis D. A., Higgins G. A., Young W. G., Goldgaber D., Gajdusek D. C., Wilson M. C., Morrison J. H. Distribution of precursor amyloid-beta-protein messenger RNA in human cerebral cortex: relationship to neurofibrillary tangles and neuritic plaques. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1691–1695. doi: 10.1073/pnas.85.5.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Multhaup G., Simms G., Pottgiesser J., Martins R. N., Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985 Nov;4(11):2757–2763. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita S., Sadlock J., Herbert J., Schon E. A. A cDNA specifying the human amyloid beta precursor protein (ABPP) encodes a 95-kDa polypeptide. Nucleic Acids Res. 1988 Oct 11;16(19):9351–9351. doi: 10.1093/nar/16.19.9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlisny M. B., Lee G., Selkoe D. J. Gene dosage of the amyloid beta precursor protein in Alzheimer's disease. Science. 1987 Oct 30;238(4827):669–671. doi: 10.1126/science.2960019. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988 Feb 11;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Robakis N. K., Ramakrishna N., Wolfe G., Wisniewski H. M. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J., Abraham C. R., Podlisny M. B., Duffy L. K. Isolation of low-molecular-weight proteins from amyloid plaque fibers in Alzheimer's disease. J Neurochem. 1986 Jun;46(6):1820–1834. doi: 10.1111/j.1471-4159.1986.tb08501.x. [DOI] [PubMed] [Google Scholar]
- Shivers B. D., Hilbich C., Multhaup G., Salbaum M., Beyreuther K., Seeburg P. H. Alzheimer's disease amyloidogenic glycoprotein: expression pattern in rat brain suggests a role in cell contact. EMBO J. 1988 May;7(5):1365–1370. doi: 10.1002/j.1460-2075.1988.tb02952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St George-Hyslop P. H., Tanzi R. E., Polinsky R. J., Neve R. L., Pollen D., Drachman D., Growdon J., Cupples L. A., Nee L., Myers R. H. Absence of duplication of chromosome 21 genes in familial and sporadic Alzheimer's disease. Science. 1987 Oct 30;238(4827):664–666. doi: 10.1126/science.2890206. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Bird E. D., Latt S. A., Neve R. L. The amyloid beta protein gene is not duplicated in brains from patients with Alzheimer's disease. Science. 1987 Oct 30;238(4827):666–669. doi: 10.1126/science.2890207. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., St George-Hyslop P. H., Haines J. L., Polinsky R. J., Nee L., Foncin J. F., Neve R. L., McClatchey A. I., Conneally P. M., Gusella J. F. The genetic defect in familial Alzheimer's disease is not tightly linked to the amyloid beta-protein gene. Nature. 1987 Sep 10;329(6135):156–157. doi: 10.1038/329156a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Broeckhoven C., Genthe A. M., Vandenberghe A., Horsthemke B., Backhovens H., Raeymaekers P., Van Hul W., Wehnert A., Gheuens J., Cras P. Failure of familial Alzheimer's disease to segregate with the A4-amyloid gene in several European families. Nature. 1987 Sep 10;329(6135):153–155. doi: 10.1038/329153a0. [DOI] [PubMed] [Google Scholar]
- Wen G. Y., Rudelli R. D., Kim K. S., Wisniewski H. M. Tangles of ependyma-choroid plexus contain B-amyloid protein epitopes and represent a new form of amyloid fiber. Arch Neurol. 1988 Dec;45(12):1298–1299. doi: 10.1001/archneur.1988.00520360016002. [DOI] [PubMed] [Google Scholar]
- Wong C. W., Quaranta V., Glenner G. G. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]