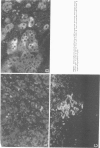
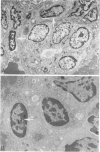
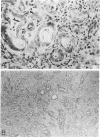
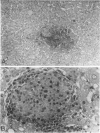
Abstract
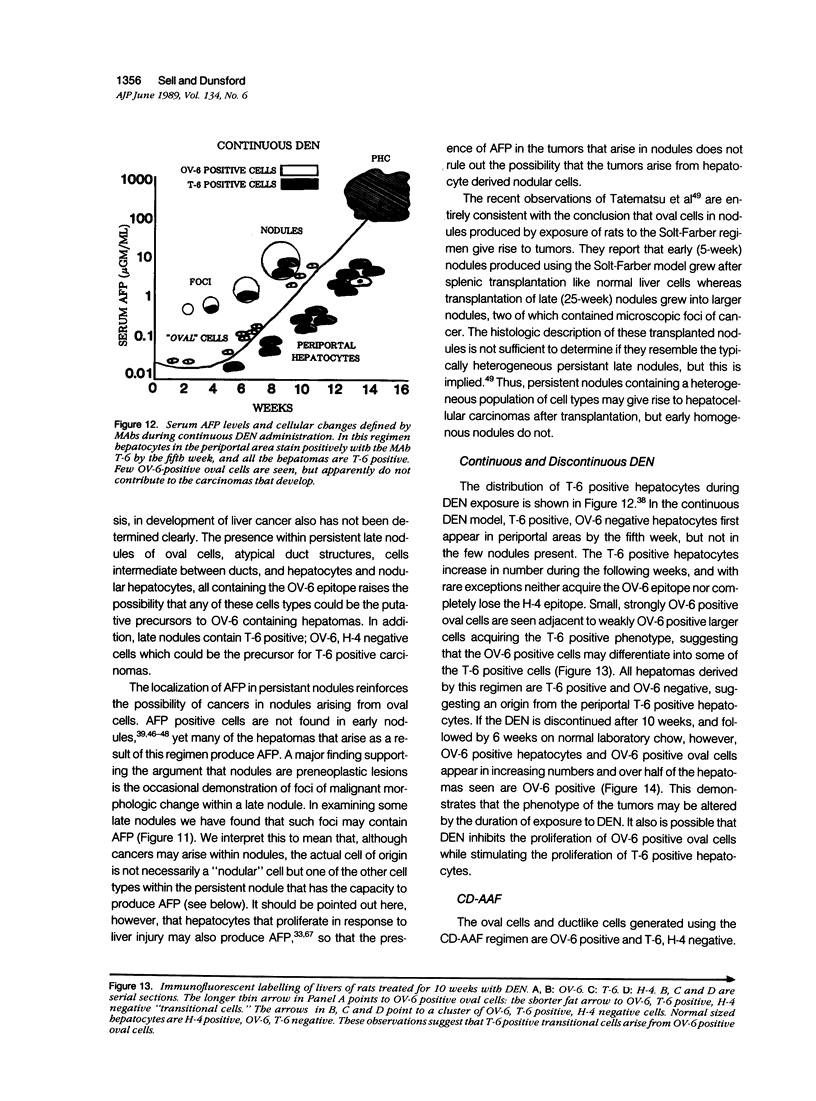
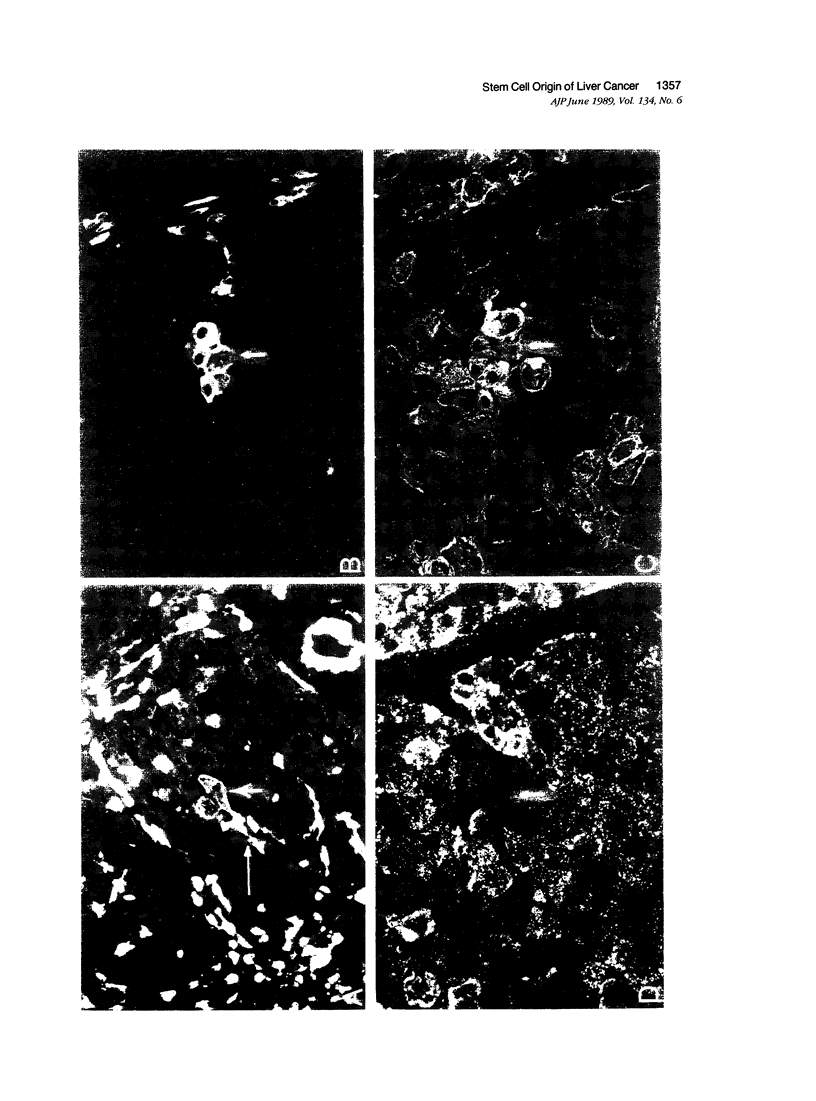
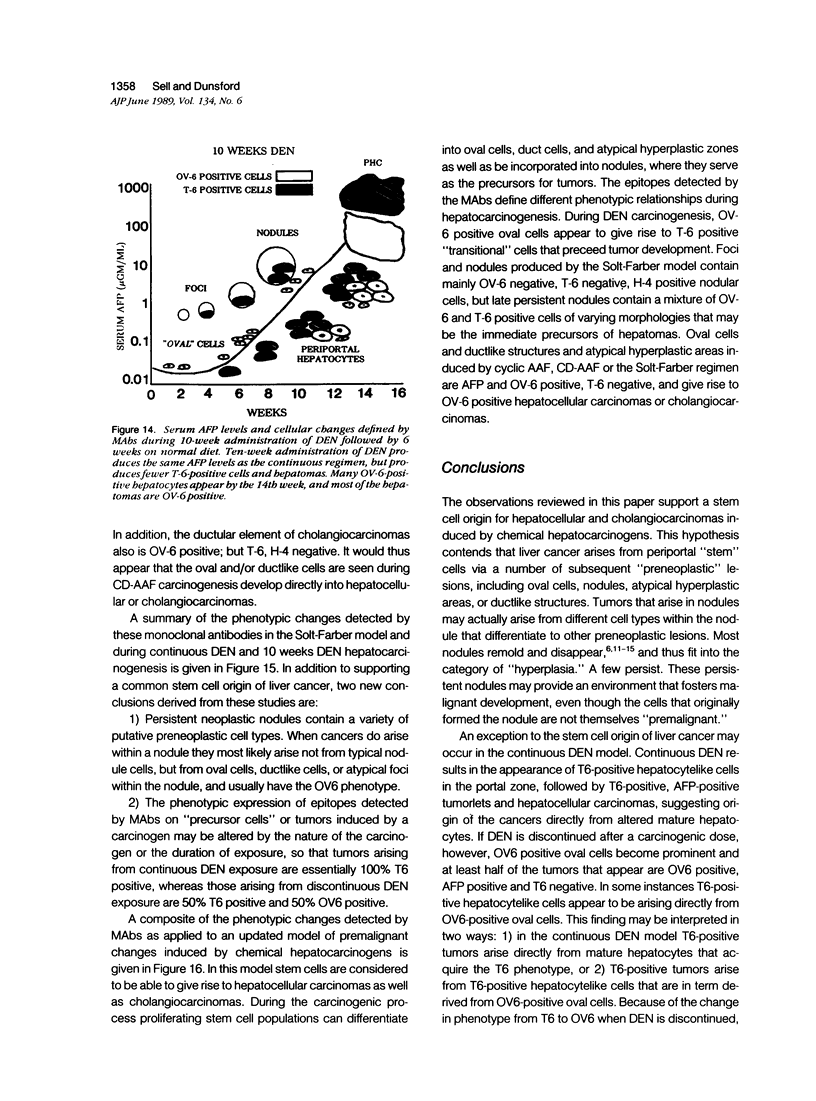
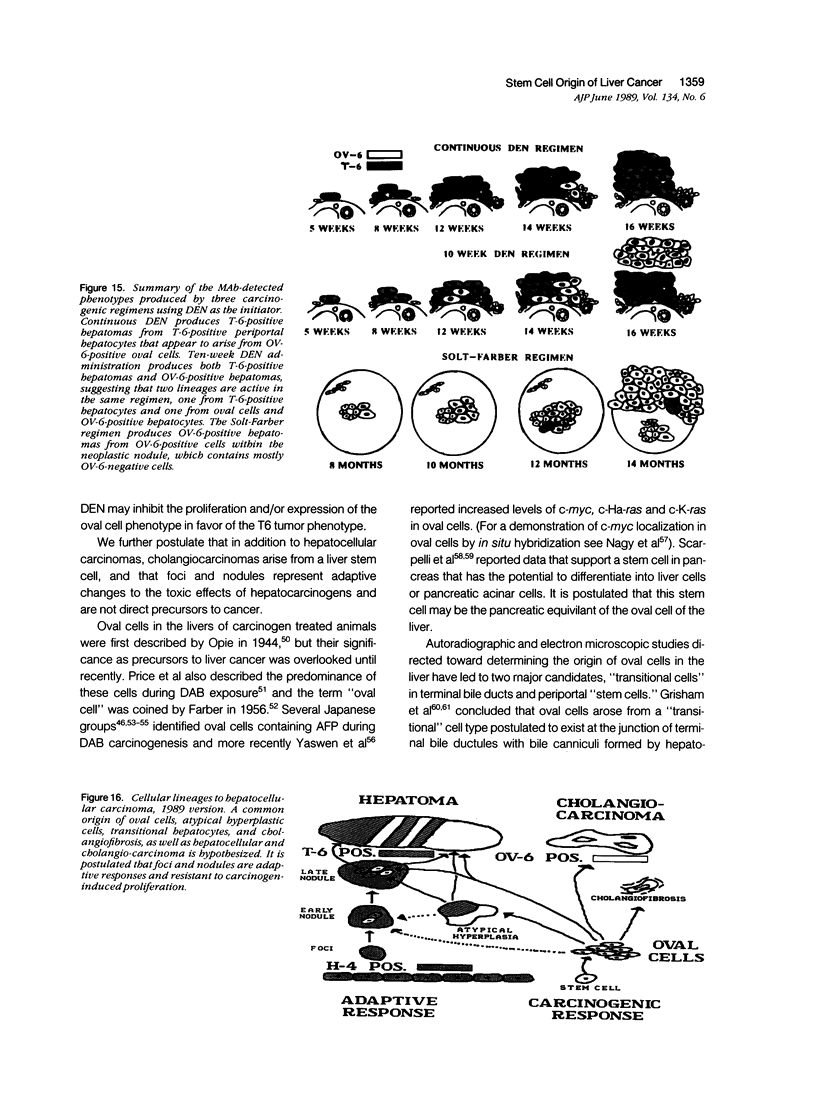
A review of the morphologic, autoradiographic, and phenotypic analysis of the cellular changes seen during induction of cancer of the liver in rats by chemical carcinogens is used to develop an alternative to the established hypothesis that chemically induced hepatocellular carcinoma arises from premalignant nodules. The authors propose that hepatocellular and ductular carcinomas arise from a pluripotent liver stem cell and that enzyme-altered foci and nodular changes are adaptive non-oncogenic responses to the toxic effects of carcinogens. It is further postulated that persistent nodules may provide an environment that nurtures development of neoplastic cells other than the altered hepatocytes that originally form the nodule. It is possible, however, that there may be more than one cellular lineage to hepatocellular cancer and that persistent nodules contain these different lineages.
Full text
PDF
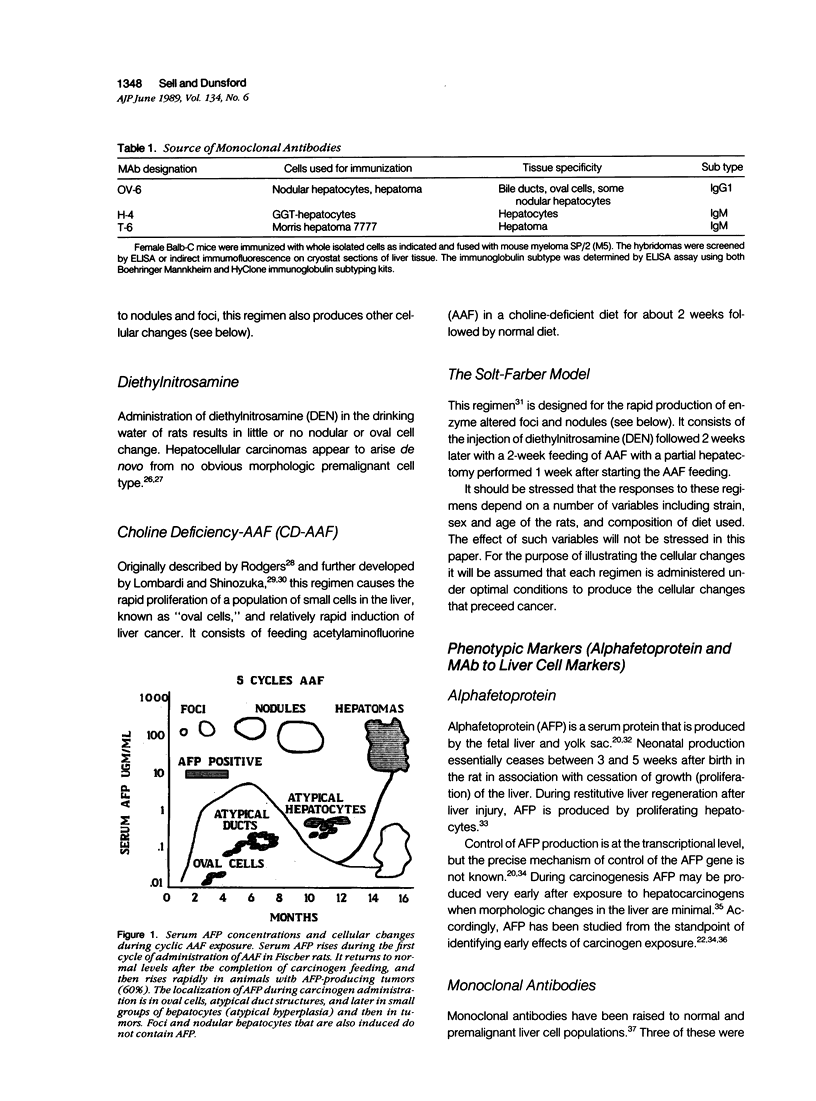
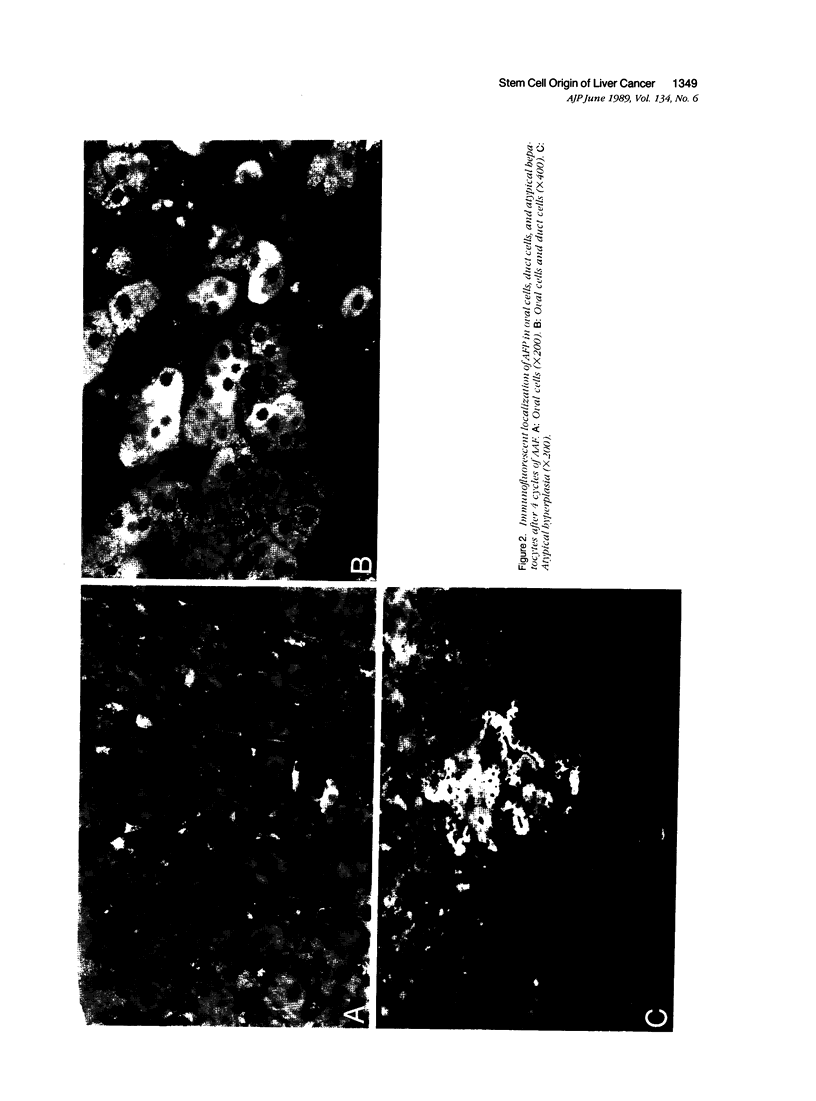
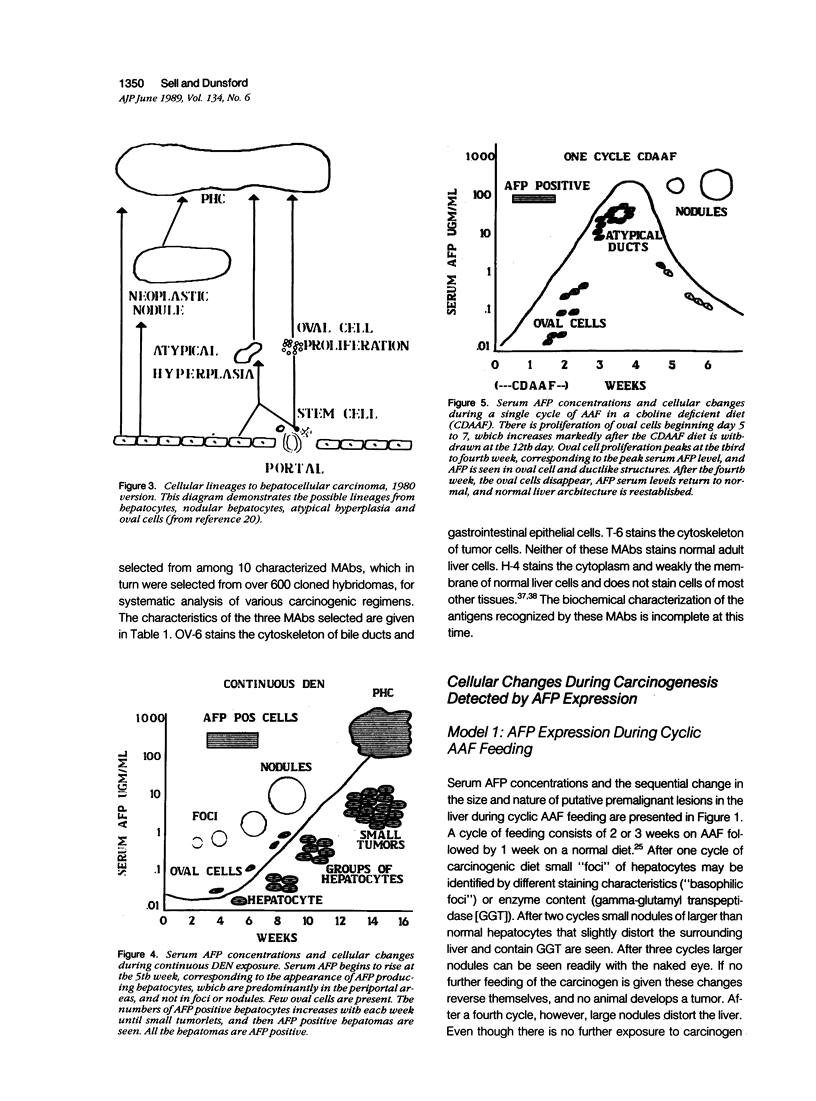
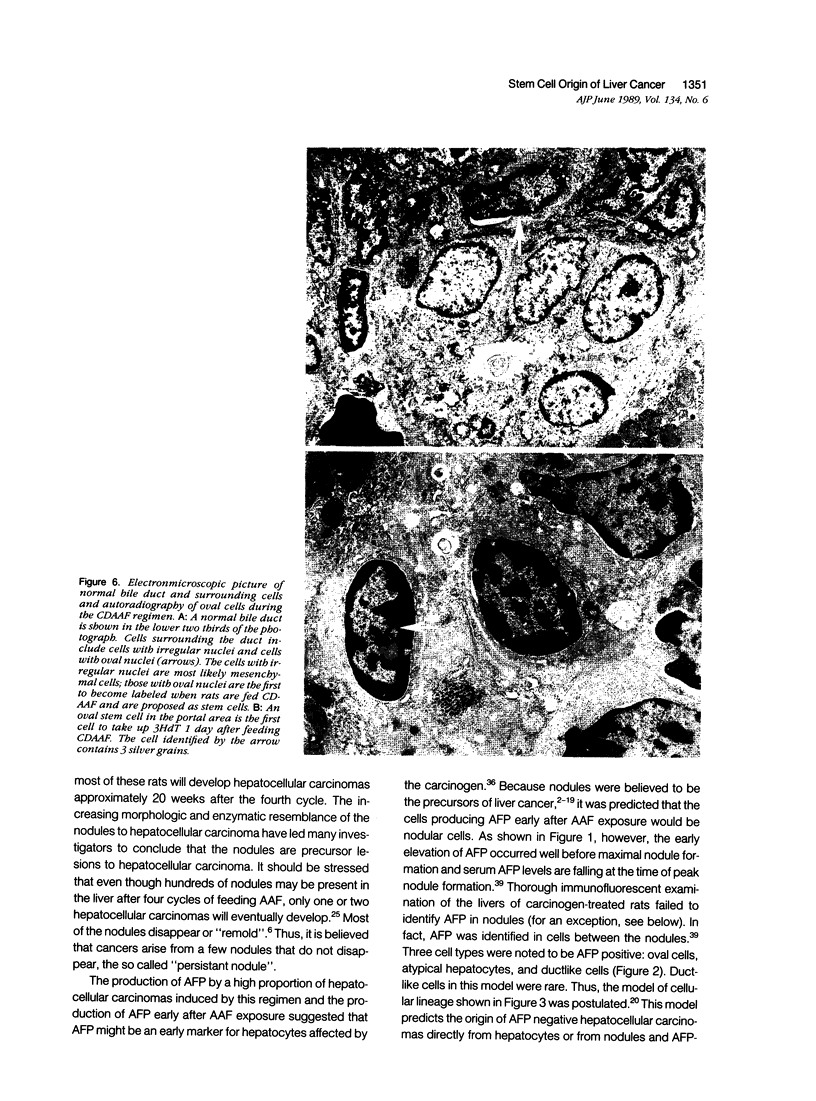
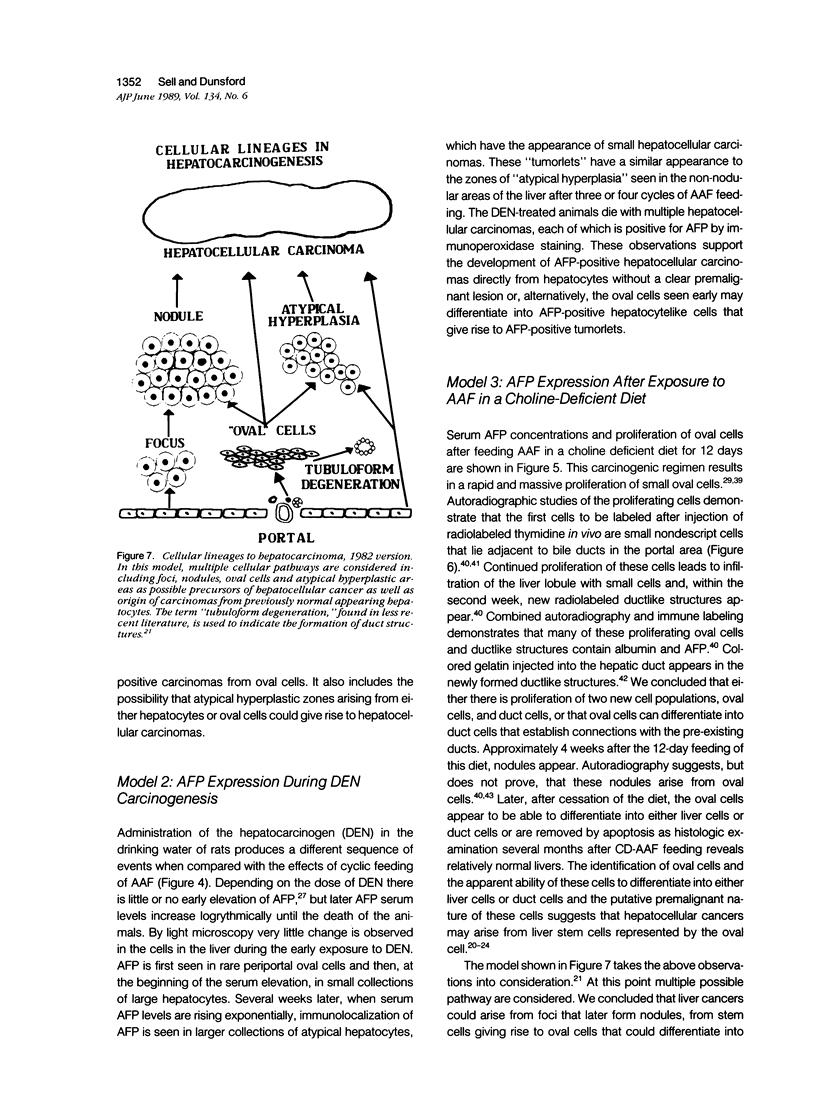
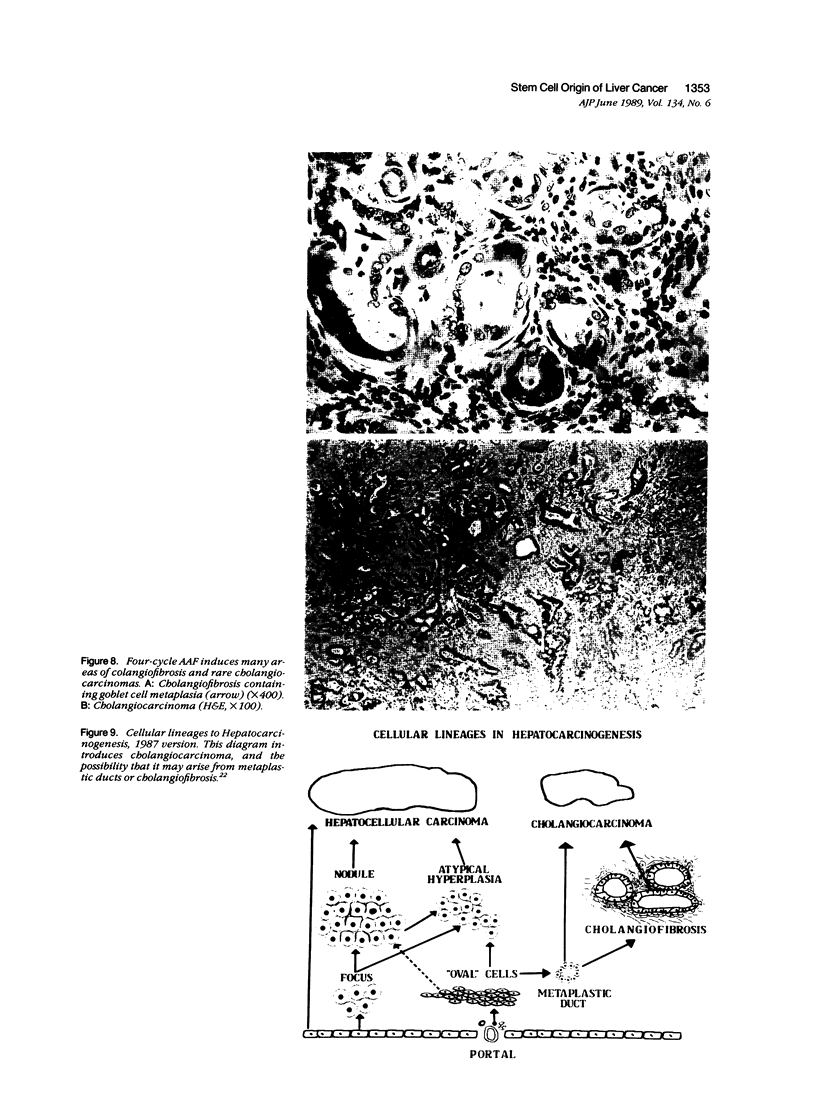
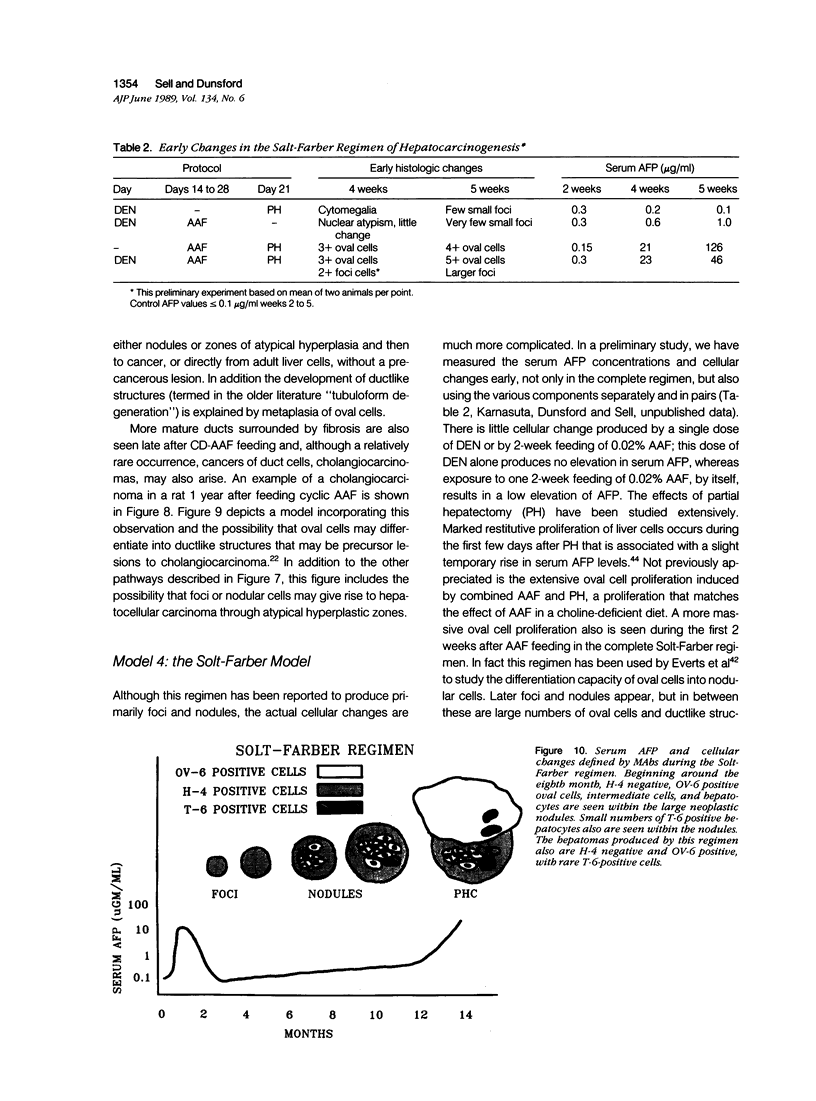





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelev G. I. Production of embryonal serum alpha-globulin by hepatomas: review of experimental and clinical data. Cancer Res. 1968 Jul;28(7):1344–1350. [PubMed] [Google Scholar]
- Bannasch P. Sequential cellular changes during chemical carcinogenesis. J Cancer Res Clin Oncol. 1984;108(1):11–22. doi: 10.1007/BF00390968. [DOI] [PubMed] [Google Scholar]
- Becker F. F., Sell S. Differences in serum alpha-fetoprotein concentrations during the carcinogenic sequences resulting from exposure to diethylnitrosamine or acetylaminofluorene. Cancer Res. 1979 May;39(5):1437–1442. [PubMed] [Google Scholar]
- Becker F. F., Sell S. Early elevation of alpha 1-fetoprotein in N-2-fluorenylacetamide hepatocarcinogenesis. Cancer Res. 1974 Oct;34(10):2489–2494. [PubMed] [Google Scholar]
- Dempo K., Chisaka N., Yoshida Y., Kaneko A., Onoé T. Immunofluorescent study on alpha-fetoprotein-producing cells in the early stage of 3'-methyl-4-dimethylaminoazobenzene carcinogenesis. Cancer Res. 1975 May;35(5):1282–1287. [PubMed] [Google Scholar]
- ELIAS H. A re-examination of the structure of the mammalian liver; the hepatic lobule and its relation to the vascular and biliary systems. Am J Anat. 1949 Nov;85(3):379-456, 15 pl. doi: 10.1002/aja.1000850303. [DOI] [PubMed] [Google Scholar]
- Engelhardt N. V., Baranov V. N., Lazareva M. N., Goussev A. I. Ultrastructural localisation of alpha-fetoprotin (AFP) in regenerating mouse liver poisoned with CCL4. 1. Reexpression of AFP in differentiated hepatocytes. Histochemistry. 1984;80(4):401–407. doi: 10.1007/BF00495425. [DOI] [PubMed] [Google Scholar]
- Engelhardt N. V., Lazareva M. N., Abelev G. I., Uryvaeva I. V., Factor V. M., Brodsky V. Y. Detection of alpha-foetoprotein in mouse liver differentiated hepatocytes before their progression through S phase. Nature. 1976 Sep 9;263(5573):146–148. doi: 10.1038/263146a0. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Marsden E., Thorgeirsson S. S. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987 Nov;8(11):1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- FARBER E. ETHIONINE CARCINOGENESIS. Adv Cancer Res. 1963;7:383–474. doi: 10.1016/s0065-230x(08)60986-0. [DOI] [PubMed] [Google Scholar]
- FARBER E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956 Feb;16(2):142–148. [PubMed] [Google Scholar]
- Farber E. Biochemistry of carcinogenesis. Cancer Res. 1968 Sep;28(9):1859–1869. [PubMed] [Google Scholar]
- Farber E., Cameron R. The sequential analysis of cancer development. Adv Cancer Res. 1980;31:125–226. doi: 10.1016/s0065-230x(08)60658-2. [DOI] [PubMed] [Google Scholar]
- Farber E. Carcinogenesis--cellular evolution as a unifying thread: Presidential address. Cancer Res. 1973 Nov;33(11):2537–2550. [PubMed] [Google Scholar]
- Farber E. Chemical carcinogenesis: a biologic perspective. Am J Pathol. 1982 Feb;106(2):271–296. [PMC free article] [PubMed] [Google Scholar]
- Farber E. Chemicals, evolution, and cancer development: Rous-Whipple Award Lecture. Am J Pathol. 1982 Sep;108(3):270–275. [PMC free article] [PubMed] [Google Scholar]
- Farber E. Pathogenesis of liver cancer. Arch Pathol. 1974 Sep;98(3):145–148. [PubMed] [Google Scholar]
- Farber E. Pre-cancerous steps in carcinogenesis. Their physiological adaptive nature. Biochim Biophys Acta. 1984;738(4):171–180. doi: 10.1016/0304-419x(83)90002-1. [DOI] [PubMed] [Google Scholar]
- Farber E., Sarma D. S. Hepatocarcinogenesis: a dynamic cellular perspective. Lab Invest. 1987 Jan;56(1):4–22. [PubMed] [Google Scholar]
- Farber E. The genesis of cancer with chemicals. Arch Pathol Lab Med. 1980 Oct;104(10):499–502. [PubMed] [Google Scholar]
- Farber E. The sequential analysis of cancer induction with chemicals. Acta Pathol Jpn. 1981 Jan;31(1):1–11. doi: 10.1111/j.1440-1827.1981.tb00978.x. [DOI] [PubMed] [Google Scholar]
- Farber E. The sequential analysis of liver cancer induction. Biochim Biophys Acta. 1980 May 6;605(2):149–166. doi: 10.1016/0304-419x(80)90002-5. [DOI] [PubMed] [Google Scholar]
- Fujita S., Ishizuka H., Kamimura N., Kaneda H., Ariga K. The alpha-fetoprotein-producing cells in the early stage of the experimental liver cancer. Ann N Y Acad Sci. 1975 Aug 22;259:217–220. doi: 10.1111/j.1749-6632.1975.tb25416.x. [DOI] [PubMed] [Google Scholar]
- GRISHAM J. W., HARTROFT W. S. Morphologic identification by electron microscopy of "oval" cells in experimental hepatic degeneration. Lab Invest. 1961 Mar-Apr;10:317–332. [PubMed] [Google Scholar]
- GRISHAM J. W., PORTA E. A. ORIGIN AND FATE OF PROLIFERATED HEPATIC DUCTAL CELLS IN THE RAT: ELECTRON MICROSCOPIC AND AUTORADIOGRAPHIC STUDIES. Exp Mol Pathol. 1964 Jun;86:242–261. doi: 10.1016/0014-4800(64)90057-7. [DOI] [PubMed] [Google Scholar]
- Germain L., Blouin M. J., Marceau N. Biliary epithelial and hepatocytic cell lineage relationships in embryonic rat liver as determined by the differential expression of cytokeratins, alpha-fetoprotein, albumin, and cell surface-exposed components. Cancer Res. 1988 Sep 1;48(17):4909–4918. [PubMed] [Google Scholar]
- Germain L., Noël M., Gourdeau H., Marceau N. Promotion of growth and differentiation of rat ductular oval cells in primary culture. Cancer Res. 1988 Jan 15;48(2):368–378. [PubMed] [Google Scholar]
- Goldsworthy T. L., Hanigan M. H., Pitot H. C. Models of hepatocarcinogenesis in the rat--contrasts and comparisons. Crit Rev Toxicol. 1986;17(1):61–89. doi: 10.3109/10408448609037071. [DOI] [PubMed] [Google Scholar]
- Grisham J. W. Cell types in long-term propagable cultures of rat liver. Ann N Y Acad Sci. 1980;349:128–137. doi: 10.1111/j.1749-6632.1980.tb29521.x. [DOI] [PubMed] [Google Scholar]
- Grisham J. W. Cell types in rat liver cultures: their identification and isolation. Mol Cell Biochem. 1983;53-54(1-2):23–33. doi: 10.1007/BF00225244. [DOI] [PubMed] [Google Scholar]
- Hixson D. C., Allison J. P. Monoclonal antibodies recognizing oval cells induced in the liver of rats by N-2-fluorenylacetamide or ethionine in a choline-deficient diet. Cancer Res. 1985 Aug;45(8):3750–3760. [PubMed] [Google Scholar]
- Iype P. T. Cultures from adult rat liver cells. I. Establishment of monolayer cell-cultures from normal liver. J Cell Physiol. 1971 Oct;78(2):281–288. doi: 10.1002/jcp.1040780217. [DOI] [PubMed] [Google Scholar]
- Koen H., Pugh T. D., Nychka D., Goldfarb S. Presence of alpha-fetoprotein-positive cells in hepatocellular foci and microcarcinomas induced by single injections of diethylnitrosamine in infant mice. Cancer Res. 1983 Feb;43(2):702–708. [PubMed] [Google Scholar]
- Kuhlmann W. D. Localization of alpha1-fetoprotein and DNA-synthesis in liver cell populations during experimental hepatocarcinogenesis in rats. Int J Cancer. 1978 Mar 15;21(3):368–380. doi: 10.1002/ijc.2910210319. [DOI] [PubMed] [Google Scholar]
- Leffert H., Moran T., Sell S., Skelly H., Ibsen K., Mueller M., Arias I. Growth state-dependent phenotypes of adult hepatocytes in primary monolayer culture. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1834–1838. doi: 10.1073/pnas.75.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty M., Das P. K., Mittal A., Nayak N. C. Cellular basis of induced alpha-fetoprotein synthesis by hepatocytes of adult mouse after hepatotoxic injury and partial hepatectomy. Int J Cancer. 1978 Aug 15;22(2):181–188. doi: 10.1002/ijc.2910220212. [DOI] [PubMed] [Google Scholar]
- Nagy P., Evarts R. P., Marsden E., Roach J., Thorgeirsson S. S. Cellular distribution of c-myc transcripts during chemical hepatocarcinogenesis in rats. Cancer Res. 1988 Oct 1;48(19):5522–5527. [PubMed] [Google Scholar]
- Onda H. Immunohistological studies on alpha1-fetoprotein and alpha1-acid glycoprotein during azo dye hepatocarcinogenesis in rats. Gan. 1976 Apr;67(2):253–262. [PubMed] [Google Scholar]
- Onoé T., Kaneko A., Dempo K., Ogawa K., Minase T. Alpha-Fetoprotein and early histological changes of hepatic tissue in DAB-hepatocarcinogenesis. Ann N Y Acad Sci. 1975 Aug 22;259:168–180. doi: 10.1111/j.1749-6632.1975.tb25412.x. [DOI] [PubMed] [Google Scholar]
- PRICE J. M., HARMAN J. W., MILLER E. C., MILLER J. A. Progressive microscopic alterations in the livers of rats fed the hepatic carcinogens 3'-methyl-4-dimethylaminoazobenzene and 4'-fluoro-4-dimethylaminoazobenzene. Cancer Res. 1952 Mar;12(3):192–200. [PubMed] [Google Scholar]
- Petropoulos C. J., Yaswen P., Panzica M., Fausto N. Cell lineages in liver carcinogenesis: possible clues from studies of the distribution of alpha-fetoprotein RNA sequences in cell populations isolated from normal, regenerating, and preneoplastic rat livers. Cancer Res. 1985 Nov;45(11 Pt 2):5762–5768. [PubMed] [Google Scholar]
- Pitot H. C., Sirica A. E. The stages of initiation and promotion in hepatocarcinogenesis. Biochim Biophys Acta. 1980 May 6;605(2):191–215. doi: 10.1016/0304-419x(80)90004-9. [DOI] [PubMed] [Google Scholar]
- Rabes H. M. Development and growth of early preneoplastic lesions induced in the liver by chemical carcinogens. J Cancer Res Clin Oncol. 1983;106(2):85–92. doi: 10.1007/BF00395384. [DOI] [PubMed] [Google Scholar]
- Rabes H. M., Wirsching R., Tuczek H. V., Iseler G. Analysis of cell cycle compartments of hepatocytes after partial hepatecomy. Cell Tissue Kinet. 1976 Nov;9(6):517–532. doi: 10.1111/j.1365-2184.1976.tb01301.x. [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Rao M. S., Qureshi S. A., Reddy M. K., Scarpelli D. G., Lalwani N. D. Induction and origin of hepatocytes in rat pancreas. J Cell Biol. 1984 Jun;98(6):2082–2090. doi: 10.1083/jcb.98.6.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A. E. Variable effects of a lipotrope-deficient, high-fat diet on chemical carcinogenesis in rats. Cancer Res. 1975 Sep;35(9):2469–2474. [PubMed] [Google Scholar]
- Scarpelli D. G. Multipotent developmental capacity of cells in the adult animal. Lab Invest. 1985 Apr;52(4):331–333. [PubMed] [Google Scholar]
- Sell S., Becker F. F. alpha-Fetoprotein. J Natl Cancer Inst. 1978 Jan;60(1):19–26. doi: 10.1093/jnci/60.1.19. [DOI] [PubMed] [Google Scholar]
- Sell S. Comparison of oval cells induced in rat liver by feeding N-2-fluorenylacetamide in a choline-devoid diet and bile duct cells induced by feeding 4,4'-diaminodiphenylmethane. Cancer Res. 1983 Apr;43(4):1761–1767. [PubMed] [Google Scholar]
- Sell S. Distribution of alpha-fetoprotein- and albumin-containing cells in the livers of Fischer rats fed four cycles of N-2-fluorenylacetamide. Cancer Res. 1978 Sep;38(9):3107–3113. [PubMed] [Google Scholar]
- Sell S. Heterogeneity of alpha-fetoprotein(AFP) and albumin containing cells in normal and pathological permissive states for AFP production: AFP containing cells induced in adult rats recapitulate the appearance of AFP containing hepatocytes in fetal rats. Oncodev Biol Med. 1980;1(2):93–105. [PubMed] [Google Scholar]
- Sell S., Hunt J. M., Knoll B. J., Dunsford H. A. Cellular events during hepatocarcinogenesis in rats and the question of premalignancy. Adv Cancer Res. 1987;48:37–111. doi: 10.1016/s0065-230x(08)60690-9. [DOI] [PubMed] [Google Scholar]
- Sell S., Leffert H. L. An evaluation of cellular lineages in the pathogenesis of experimental hepatocellular carcinoma. Hepatology. 1982 Jan-Feb;2(1):77–86. doi: 10.1002/hep.1840020113. [DOI] [PubMed] [Google Scholar]
- Sell S., Leffert H. L., Shinozuka H., Lombardi B., Gochman N. Rapid development of large numbers of alpha-fetoprotein-containing "oval" cells in the liver of rats fed N-2-fluorenylacetamide in a choline-devoid diet. Gan. 1981 Aug;72(4):479–487. [PubMed] [Google Scholar]
- Sell S., Nichols M., Becker F. F., Leffert H. L. Hepatocyte proliferation and alpha 1-fetoprotein in pregnant, neonatal, and partially hepatectomized rats. Cancer Res. 1974 Apr;34(4):865–871. [PubMed] [Google Scholar]
- Sell S., Osborn K., Leffert H. L. Autoradiography of "oval cells" appearing rapidly in the livers of rats fed N-2-fluorenylacetamide in a choline devoid diet. Carcinogenesis. 1981;2(1):7–14. doi: 10.1093/carcin/2.1.7. [DOI] [PubMed] [Google Scholar]
- Sell S., Sala-Trepat J. M., Sargent T. D., Thomas K., Nahon J. L., Goodman T. A., Bonner J. Molecular mechanisms of control of albumin and alphafetoprotein production: a system to study the early effects of chemical hepatocarcinogens. Cell Biol Int Rep. 1980 Mar;4(3):235–254. doi: 10.1016/0309-1651(80)90056-9. [DOI] [PubMed] [Google Scholar]
- Sell S., Salman J. Light- and electron-microscopic autoradiographic analysis of proliferating cells during the early stages of chemical hepatocarcinogenesis in the rat induced by feeding N-2-fluorenylacetamide in a choline-deficient diet. Am J Pathol. 1984 Feb;114(2):287–300. [PMC free article] [PubMed] [Google Scholar]
- Sells M. A., Katyal S. L., Shinozuka H., Estes L. W., Sell S., Lombardi B. Isolation of oval cells and transitional cells from the livers of rats fed the carcinogen DL-ethionine. J Natl Cancer Inst. 1981 Feb;66(2):355–362. [PubMed] [Google Scholar]
- Shinozuka H., Lombardi B., Sell S., Iammarino R. M. Enhancement of DL-ethionine-induced liver carcinogenesis in rats fed a choline-devoid diet. J Natl Cancer Inst. 1978 Sep;61(3):813–817. [PubMed] [Google Scholar]
- Solt D. B., Medline A., Farber E. Rapid emergence of carcinogen-induced hyperplastic lesions in a new model for the sequential analysis of liver carcinogenesis. Am J Pathol. 1977 Sep;88(3):595–618. [PMC free article] [PubMed] [Google Scholar]
- Tatematsu M., Lee G., Hayes M. A., Farber E. Progression in hepatocarcinogenesis: differences in growth and behavior of transplants of early and later hepatocyte nodules in the rat spleen. Cancer Res. 1987 Sep 1;47(17):4699–4705. [PubMed] [Google Scholar]
- Tchipysheva T. A., Guelstein V. I., Bannikov G. A. alpha-fetoprotein-containing cells in the early stages of liver carcinogenesis induced by 3'-methyl-4-dimethyl-aminoazobenzene and 2-acetylaminofluorene. Int J Cancer. 1977 Sep 15;20(3):388–393. doi: 10.1002/ijc.2910200310. [DOI] [PubMed] [Google Scholar]
- Teebor G. W., Becker F. F. Regression and persistence of hyperplastic hepatic nodules induced by N-2-Fluorenylacetamide and their relationship to hepatocarcinogenesis. Cancer Res. 1971 Jan;31(1):1–3. [PubMed] [Google Scholar]
- Tournier I., Legrès L., Schoevaert D., Feldmann G., Bernuau D. Cellular analysis of alpha-fetoprotein gene activation during carbon tetrachloride and D-galactosamine-induced acute liver injury in rats. Lab Invest. 1988 Nov;59(5):657–665. [PubMed] [Google Scholar]
- Tsao M. S., Grisham J. W. Hepatocarcinomas, cholangiocarcinomas, and hepatoblastomas produced by chemically transformed cultured rat liver epithelial cells. A light- and electron-microscopic analysis. Am J Pathol. 1987 Apr;127(1):168–181. [PMC free article] [PubMed] [Google Scholar]
- WILSON J. W., LEDUC E. H. Role of cholangioles in restoration of the liver of the mouse after dietary injury. J Pathol Bacteriol. 1958 Oct;76(2):441–449. doi: 10.1002/path.1700760213. [DOI] [PubMed] [Google Scholar]
- Williams G. M. The pathogenesis of rat liver cancer caused by chemical carcinogens. Biochim Biophys Acta. 1980 May 6;605(2):167–189. doi: 10.1016/0304-419x(80)90003-7. [DOI] [PubMed] [Google Scholar]
- Yaswen P., Goyette M., Shank P. R., Fausto N. Expression of c-Ki-ras, c-Ha-ras, and c-myc in specific cell types during hepatocarcinogenesis. Mol Cell Biol. 1985 Apr;5(4):780–786. doi: 10.1128/mcb.5.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S., Satoh M., Lombardi B. Bile ductular cells and the phenotypic heterogeneity of the population of hepatic non-parenchymal epithelial cells induced in rats by chemical carcinogens. Carcinogenesis. 1986 Jul;7(7):1215–1219. doi: 10.1093/carcin/7.7.1215. [DOI] [PubMed] [Google Scholar]
- Yoshimura H., Harris R., Yokoyama S., Takahashi S., Sells M. A., Pan S. F., Lombardi B. Anaplastic carcinomas in nude mice and in original donor strain rats inoculated with cultured oval cells. Am J Pathol. 1983 Mar;110(3):322–332. [PMC free article] [PubMed] [Google Scholar]