Abstract
The low (NF-L) and middle (NF-M) molecular weight (Mr) neurofilament (NF) subunits are expressed before the high (NF-H) Mr NF subunit in embryonic neurons. Thereafter, NF-M attains its mature state of phosphorylation more rapidly than does NF-H. However, little is known about NF subunit expression during cell division. A rapidly dividing medulloblastoma cell line (D283 MED), therefore, was examined using flow cytometry, immunochemistry, and a large panel of NF subunit-specific polyclonal and monoclonal antibodies. Many of the monoclonal antibodies (MAbs) distinguished NF-H and NF-M in different states of phosphorylation. By flow cytometry, more than 90% of the D283 cells expressed NF-H and NF-M in different states of phosphorylation, and an antiserum specific for the carboxy terminus of NF-L labeled more than 60% of these cells. Furthermore, the fluorescence intensity produced by MAbs that detected phosphorylated versus nonphosphorylated NF-H and/or NF-M epitopes, appropriately decreased or increased, respectively, by preincubating the D283 cells with alkaline phosphatase. In contrast, cell staining with antibodies specific for phosphate-independent NF protein epitopes did not change substantially as a result of enzymatic dephosphorylation. These results agreed closely with those obtained from studies of normal human spinal cord NF extracts. However, NF-H, NF-M, and NF-L were expressed throughout the cell cycle in dual parameter studies of D283 cells labeled with an antibody and propidium iodide. Nevertheless, reductions in the fluorescence intensity produced with most of these antibodies late in the cell cycle suggested that NF proteins may be subject to modifications in their structure or accessibility to antibody probes during different phases of the cell cycle. These data led to the conclusion that NF subunits are expressed throughout the cell cycle in cultured human medulloblastoma cells, but that subtle changes in the immunoreactivity of these proteins occur during cell division.
Full text
PDF




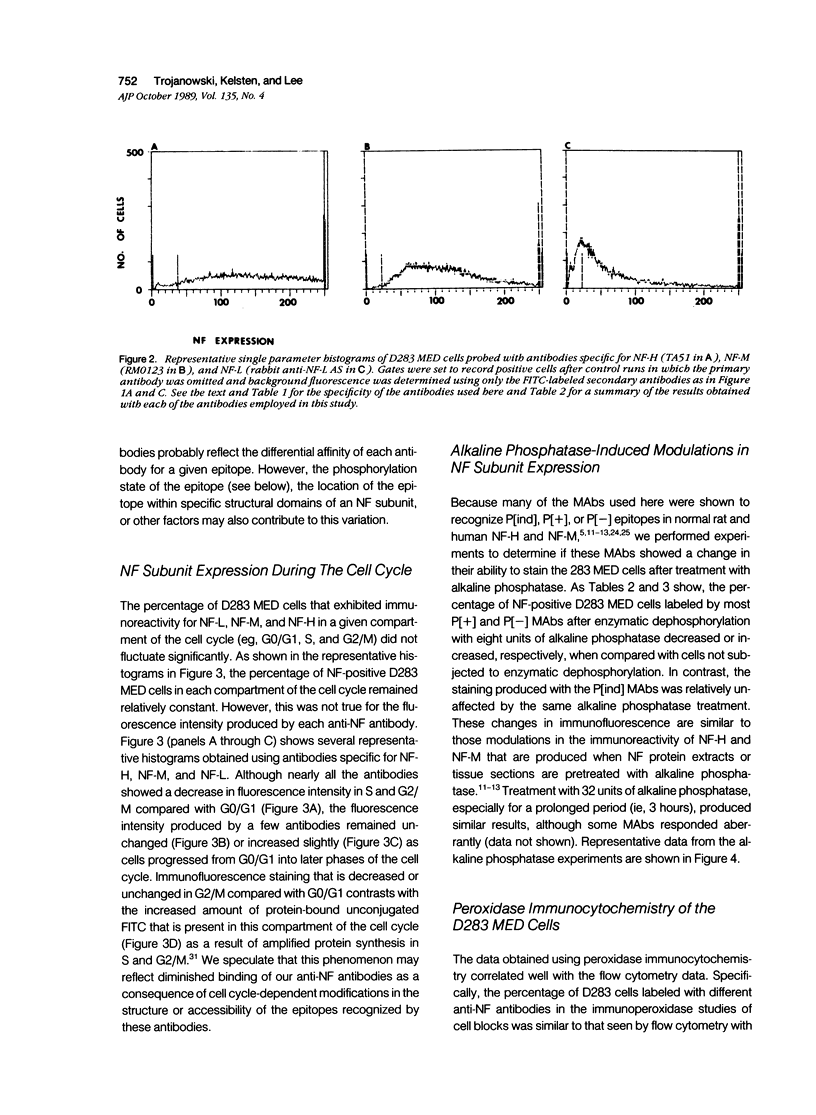






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett G. S. Changes in intermediate filament composition during neurogenesis. Curr Top Dev Biol. 1987;21:151–183. doi: 10.1016/s0070-2153(08)60136-2. [DOI] [PubMed] [Google Scholar]
- Black M. M., Lee V. M. Phosphorylation of neurofilament proteins in intact neurons: demonstration of phosphorylation in cell bodies and axons. J Neurosci. 1988 Sep;8(9):3296–3305. doi: 10.1523/JNEUROSCI.08-09-03296.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden M. J., Schlaepfer W. W., Lee V. M. The structure, biochemical properties, and immunogenicity of neurofilament peripheral regions are determined by phosphorylation state. J Biol Chem. 1985 Aug 15;260(17):9805–9817. [PubMed] [Google Scholar]
- Carden M. J., Trojanowski J. Q., Schlaepfer W. W., Lee V. M. Two-stage expression of neurofilament polypeptides during rat neurogenesis with early establishment of adult phosphorylation patterns. J Neurosci. 1987 Nov;7(11):3489–3504. doi: 10.1523/JNEUROSCI.07-11-03489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis J. E., Fey S. J., Larsen P. M., Celis A. Preferential phosphorylation of keratins and vimentin during mitosis in normal and transformed human amnion cells. Ann N Y Acad Sci. 1985;455:268–281. doi: 10.1111/j.1749-6632.1985.tb50417.x. [DOI] [PubMed] [Google Scholar]
- Cochard P., Paulin D. Initial expression of neurofilaments and vimentin in the central and peripheral nervous system of the mouse embryo in vivo. J Neurosci. 1984 Aug;4(8):2080–2094. doi: 10.1523/JNEUROSCI.04-08-02080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crissman H. A., Steinkamp J. A. Rapid, simultaneous measurement of DNA, protein, and cell volume in single cells from large mammalian cell populations. J Cell Biol. 1973 Dec;59(3):766–771. doi: 10.1083/jcb.59.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. Phosphorylation of vimentin in mitotically selected cells. In vitro cyclic AMP-independent kinase and calcium-stimulated phosphatase activities. J Cell Biol. 1989 Jan;108(1):67–78. doi: 10.1083/jcb.108.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. S., Burger P. C., Bigner S. H., Trojanowski J. Q., Brodeur G. M., He X. M., Wikstrand C. J., Kurtzberg J., Berens M. E., Halperin E. C. Phenotypic and genotypic analysis of a human medulloblastoma cell line and transplantable xenograft (D341 Med) demonstrating amplification of c-myc. Am J Pathol. 1988 Mar;130(3):472–484. [PMC free article] [PubMed] [Google Scholar]
- Friedman H. S., Burger P. C., Bigner S. H., Trojanowski J. Q., Wikstrand C. J., Halperin E. C., Bigner D. D. Establishment and characterization of the human medulloblastoma cell line and transplantable xenograft D283 Med. J Neuropathol Exp Neurol. 1985 Nov;44(6):592–605. doi: 10.1097/00005072-198511000-00005. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. Phosphorylation of desmin in vitro inhibits formation of intermediate filaments; identification of three kinase A sites in the aminoterminal head domain. EMBO J. 1988 Jan;7(1):15–20. doi: 10.1002/j.1460-2075.1988.tb02778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz N. The use of FITC-conjugated monoclonal antibodies for determination of S-phase cells with fluorescence microscopy. Cytometry. 1985 Nov;6(6):597–601. doi: 10.1002/cyto.990060615. [DOI] [PubMed] [Google Scholar]
- He X. M., Skapek S. X., Wikstrand C. J., Friedman H. S., Trojanowski J. Q., Kemshead J. T., Coakham H. B., Bigner S. H., Bigner D. D. Phenotypic analysis of four human medulloblastoma cell lines and transplantable xenografts. J Neuropathol Exp Neurol. 1989 Jan;48(1):48–68. doi: 10.1097/00005072-198901000-00005. [DOI] [PubMed] [Google Scholar]
- Hoffman P. N., Thompson G. W., Griffin J. W., Price D. L. Changes in neurofilament transport coincide temporally with alterations in the caliber of axons in regenerating motor fibers. J Cell Biol. 1985 Oct;101(4):1332–1340. doi: 10.1083/jcb.101.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M., Nishi Y., Nishizawa K., Matsuyama M., Sato C. Site-specific phosphorylation induces disassembly of vimentin filaments in vitro. Nature. 1987 Aug 13;328(6131):649–652. doi: 10.1038/328649a0. [DOI] [PubMed] [Google Scholar]
- Jacobsen P. F., Jenkyn D. J., Papadimitriou J. M. Establishment of a human medulloblastoma cell line and its heterotransplantation into nude mice. J Neuropathol Exp Neurol. 1985 Sep;44(5):472–485. doi: 10.1097/00005072-198509000-00003. [DOI] [PubMed] [Google Scholar]
- Julien J. P., Grosveld F., Yazdanbaksh K., Flavell D., Meijer D., Mushynski W. The structure of a human neurofilament gene (NF-L): a unique exon-intron organization in the intermediate filament gene family. Biochim Biophys Acta. 1987 Jun 6;909(1):10–20. doi: 10.1016/0167-4781(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Schlaepfer W. W. Structural similarities and differences between neurofilament proteins from five different species as revealed using monoclonal antibodies. J Neurosci. 1986 Aug;6(8):2179–2186. doi: 10.1523/JNEUROSCI.06-08-02179.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Trojanowski J. Q. Novel monoclonal antibodies provide evidence for the in situ existence of a nonphosphorylated form of the largest neurofilament subunit. J Neurosci. 1986 Mar;6(3):850–858. doi: 10.1523/JNEUROSCI.06-03-00850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Otvos L., Jr, Carden M. J., Hollosi M., Dietzschold B., Lazzarini R. A. Identification of the major multiphosphorylation site in mammalian neurofilaments. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1998–2002. doi: 10.1073/pnas.85.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Otvos L., Jr, Schmidt M. L., Trojanowski J. Q. Alzheimer disease tangles share immunological similarities with multiphosphorylation repeats in the two large neurofilament proteins. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7384–7388. doi: 10.1073/pnas.85.19.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Page C. D., Wu H. L., Schlaepfer W. W. Monoclonal antibodies to gel-excised glial filament protein and their reactivities with other intermediate filament proteins. J Neurochem. 1984 Jan;42(1):25–32. doi: 10.1111/j.1471-4159.1984.tb09692.x. [DOI] [PubMed] [Google Scholar]
- Lee V., Wu H. L., Schlaepfer W. W. Monoclonal antibodies recognize individual neurofilament triplet proteins. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6089–6092. doi: 10.1073/pnas.79.19.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A. Neurofilament protein phosphorylation--where, when and why. Trends Neurosci. 1988 Jul;11(7):291–292. doi: 10.1016/0166-2236(88)90086-0. [DOI] [PubMed] [Google Scholar]
- Pleasure S. J., Selzer M. E., Lee V. M. Lamprey neurofilaments combine in one subunit the features of each mammalian NF triplet protein but are highly phosphorylated only in large axons. J Neurosci. 1989 Feb;9(2):698–709. doi: 10.1523/JNEUROSCI.09-02-00698.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessmann U., Velasco M. E., Gambetti P., Autilio-Gambetti L. Neuronal and astrocytic differentiation in human neuroepithelial neoplasms. An immunohistochemical study. J Neuropathol Exp Neurol. 1983 Mar;42(2):113–121. doi: 10.1097/00005072-198303000-00001. [DOI] [PubMed] [Google Scholar]
- Rorke L. B., Gilles F. H., Davis R. L., Becker L. E. Revision of the World Health Organization classification of brain tumors for childhood brain tumors. Cancer. 1985 Oct 1;56(7 Suppl):1869–1886. doi: 10.1002/1097-0142(19851001)56:7+<1869::aid-cncr2820561330>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Rubinstein L. J. A commentary on the proposed revision of the World Health Organization classification of brain tumors for childhood brain tumors. Cancer. 1985 Oct 1;56(7 Suppl):1887–1888. doi: 10.1002/1097-0142(19851001)56:7+<1887::aid-cncr2820561331>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Schlaepfer W. W. Neurofilaments: structure, metabolism and implications in disease. J Neuropathol Exp Neurol. 1987 Mar;46(2):117–129. [PubMed] [Google Scholar]
- Schmidt M. L., Carden M. J., Lee V. M., Trojanowski J. Q. Phosphate dependent and independent neurofilament epitopes in the axonal swellings of patients with motor neuron disease and controls. Lab Invest. 1987 Mar;56(3):282–294. [PubMed] [Google Scholar]
- Schwob J. E., Farber N. B., Gottlieb D. I. Neurons of the olfactory epithelium in adult rats contain vimentin. J Neurosci. 1986 Jan;6(1):208–217. doi: 10.1523/JNEUROSCI.06-01-00208.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Weber K. Differential expression of neurofilament triplet proteins in brain development. Nature. 1982 Jul 15;298(5871):277–279. doi: 10.1038/298277a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton M. R., Darling J., Pilkington G. J., Lantos P. L., Reeves B. R., Cooper C. S. Characterization of the human cell line TE671. Carcinogenesis. 1989 May;10(5):899–905. doi: 10.1093/carcin/10.5.899. [DOI] [PubMed] [Google Scholar]
- Tremblay G. F., Lee V. M., Trojanowski J. Q. Expression of vimentin, glial filament, and neurofilament proteins in primitive childhood brain tumors. A comparative immunoblot and immunoperoxidase study. Acta Neuropathol. 1985;68(3):239–244. doi: 10.1007/BF00690201. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Friedman H. S., Burger P. C., Bigner D. D. A rapidly dividing human medulloblastoma cell line (D283 MED) expresses all three neurofilament subunits. Am J Pathol. 1987 Feb;126(2):358–363. [PMC free article] [PubMed] [Google Scholar]
- Trojanowski J. Q., Schuck T., Schmidt M. L., Lee V. M. Distribution of phosphate-independent MAP2 epitopes revealed with monoclonal antibodies in microwave-denatured human nervous system tissues. J Neurosci Methods. 1989 Aug;29(2):171–180. doi: 10.1016/0165-0270(89)90030-7. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Schuck T., Schmidt M. L., Lee V. M. Distribution of tau proteins in the normal human central and peripheral nervous system. J Histochem Cytochem. 1989 Feb;37(2):209–215. doi: 10.1177/37.2.2492045. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Walkenstein N., Lee V. M. Expression of neurofilament subunits in neurons of the central and peripheral nervous system: an immunohistochemical study with monoclonal antibodies. J Neurosci. 1986 Mar;6(3):650–660. doi: 10.1523/JNEUROSCI.06-03-00650.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachnis A. T., Trojanowski J. Q., Memmo M., Schlaepfer W. W. Expression of neurofilament proteins in the hypertrophic granule cells of Lhermitte-Duclos disease: an explanation for the mass effect and the myelination of parallel fibers in the disease state. J Neuropathol Exp Neurol. 1988 May;47(3):206–216. doi: 10.1097/00005072-198805000-00002. [DOI] [PubMed] [Google Scholar]



