Abstract
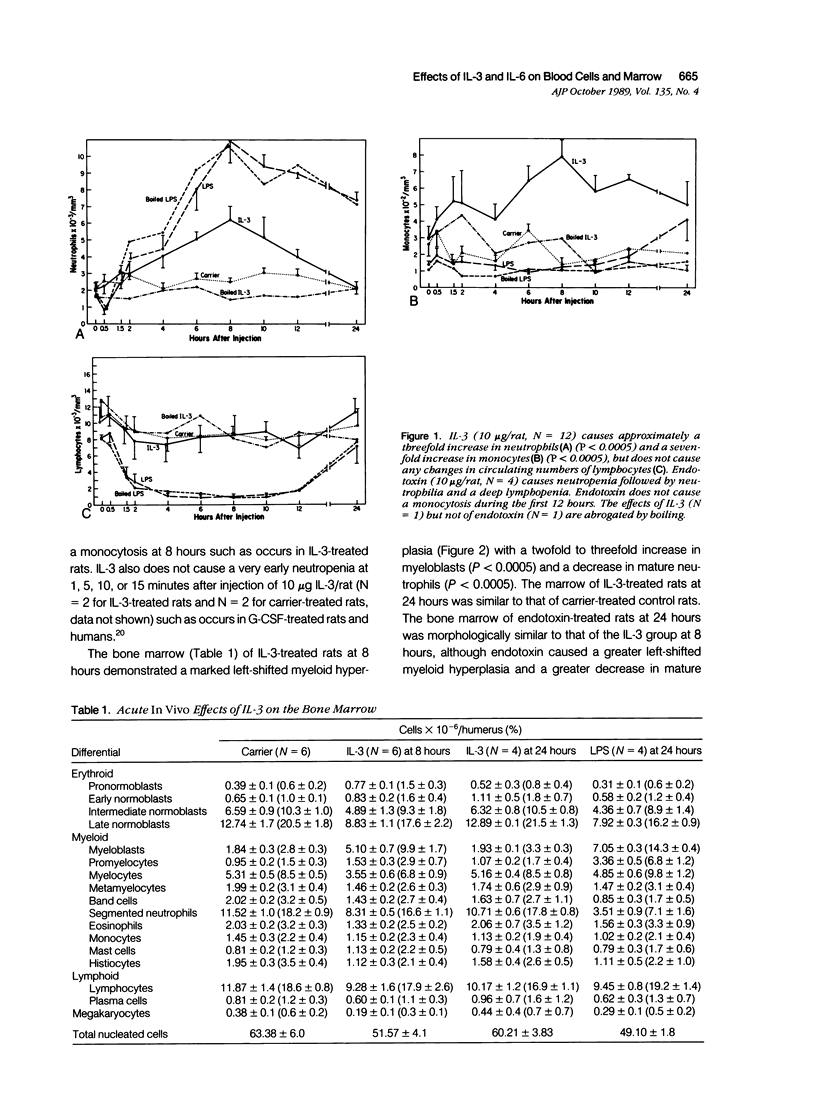
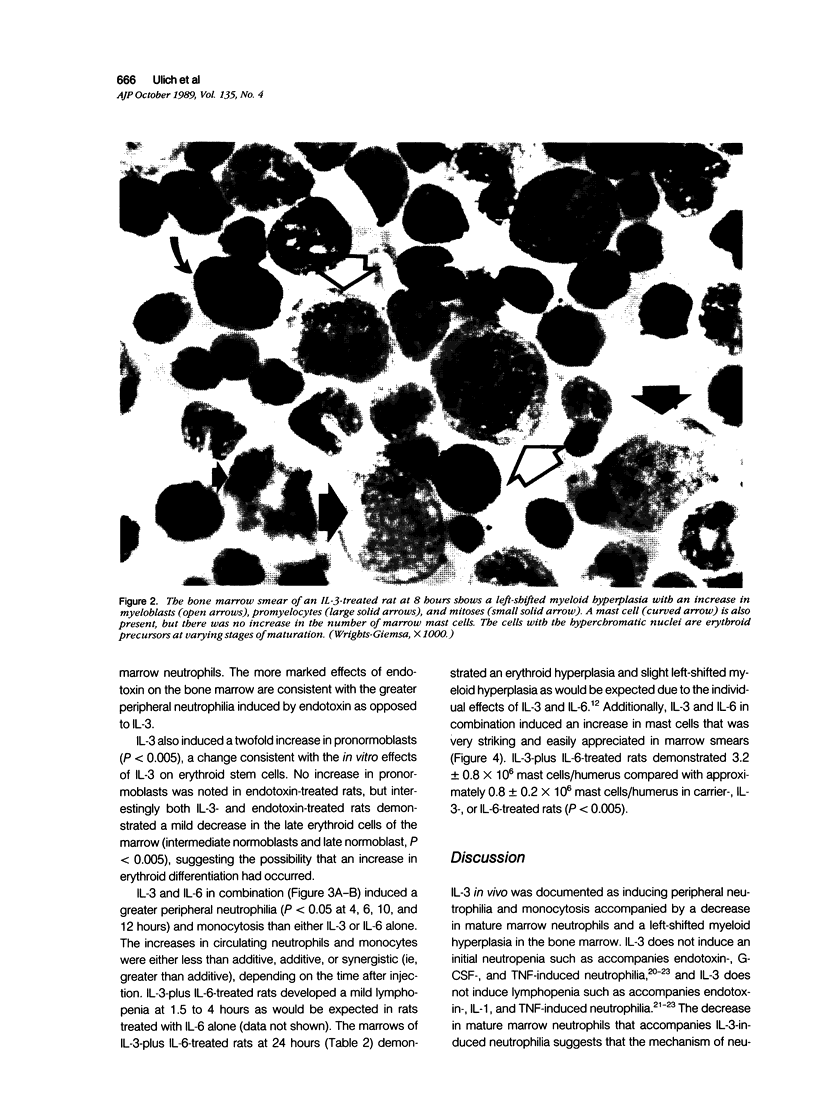
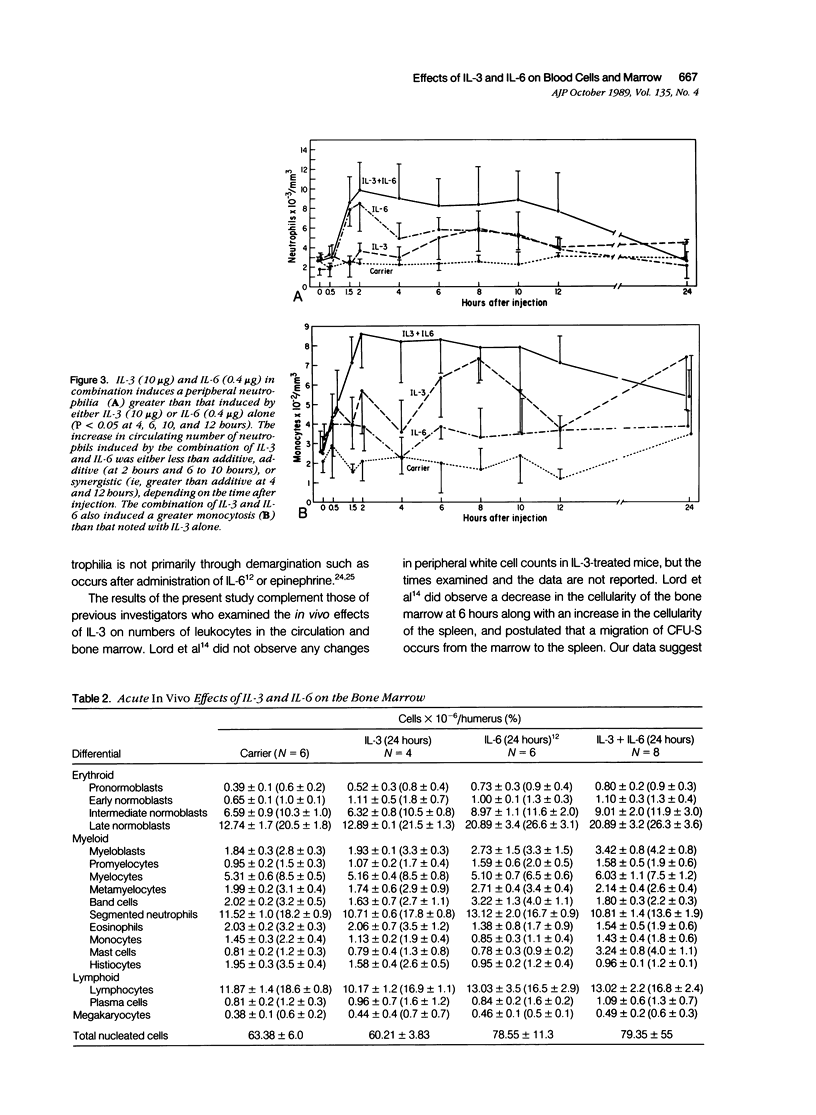
Recombinant human IL-3 administered intravenously to rats as a single injection induced peripheral neutrophilia and monocytosis beginning at 4 to 6 hours after injection, peaking at 8 hours, and subsiding to normal by 12 to 24 hours. IL-3 did not induce an initial neutropenia such as accompanies endotoxin-, G-CSF-, and TNF-induced neutrophilia, or lymphopenia such as accompanies endotoxin-, IL-1-, and TNF-induced neutrophilia. The IL-3-induced peripheral neutrophilia was accompanied by a decrease in mature marrow neutrophils, indicating that the mechanism of neutrophilia was through marrow release rather than by demargination, which occurs after the administration of epinephrine or IL-6. The release of mature marrow neutrophils further suggests that IL-3 either has intrinsic neutrophil releasing activity or indirectly causes neutrophil release through the gene expression of a second cytokine. IL-3 induced a striking left-shifted myeloid hyperplasia in the bone marrow at 8 hours that morphologically was very similar to that observed after administration of endotoxin, a finding consistent with the hypothesis of previous investigators that endotoxin may in part act indirectly on hematopoietic cells by eliciting local marrow production of IL-3. Finally, IL-3 induced an increase in marrow pronormoblasts at 8 hours, consistent with the in vitro proliferative effect of IL-3 on erythroid stem cells. The combination of IL-3 and IL-6 induced a synergistic peripheral neutrophilia and monocytosis and a striking synergistic increase in marrow mast cells. The combination of IL-3 and IL-6 also induced an erythroid and left-shifted myeloid hyperplasia such as would be expected given the individual effects of these hematopoietic growth factors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bot F. J., Dorssers L., Wagemaker G., Löwenberg B. Stimulating spectrum of human recombinant multi-CSF (IL-3) on human marrow precursors: importance of accessory cells. Blood. 1988 Jun;71(6):1609–1614. [PubMed] [Google Scholar]
- Broxmeyer H. E., Williams D. E., Cooper S., Shadduck R. K., Gillis S., Waheed A., Urdal D. L., Bicknell D. C. Comparative effects in vivo of recombinant murine interleukin 3, natural murine colony-stimulating factor-1, and recombinant murine granulocyte-macrophage colony-stimulating factor on myelopoiesis in mice. J Clin Invest. 1987 Mar;79(3):721–730. doi: 10.1172/JCI112877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno E., Briddell R., Hoffman R. Effect of recombinant and purified hematopoietic growth factors on human megakaryocyte colony formation. Exp Hematol. 1988 Jun;16(5):371–377. [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R., Marsh J. C., Cartwright G. E., Wintrobe M. M. Quantitative studies of blood and bone marrow neutrophils in normal mice. Am J Physiol. 1968 Aug;215(2):353–360. doi: 10.1152/ajplegacy.1968.215.2.353. [DOI] [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Donahue R. E., Wang E. A., Stone D. K., Kamen R., Wong G. G., Sehgal P. K., Nathan D. G., Clark S. C. Stimulation of haematopoiesis in primates by continuous infusion of recombinant human GM-CSF. 1986 Jun 26-Jul 2Nature. 321(6073):872–875. doi: 10.1038/321872a0. [DOI] [PubMed] [Google Scholar]
- Fabian I., Bleiberg I., Riklis I., Kletter Y. Enhanced reconstitution of hematopoietic organs in irradiated mice, following their transplantation with bone marrow cells pretreated with recombinant interleukin 3. Exp Hematol. 1987 Dec;15(11):1140–1144. [PubMed] [Google Scholar]
- HULSE E. V. QUANTITATIVE CELL COUNTS OF THE BONE MARROW AND BLOOD AND THEIR SECULAR VARIATIONS IN THE NORMAL ADULT RAT. Acta Haematol. 1964 Jan;31:50–63. doi: 10.1159/000209613. [DOI] [PubMed] [Google Scholar]
- Helfgott D. C., May L. T., Sthoeger Z., Tamm I., Sehgal P. B. Bacterial lipopolysaccharide (endotoxin) enhances expression and secretion of beta 2 interferon by human fibroblasts. J Exp Med. 1987 Nov 1;166(5):1300–1309. doi: 10.1084/jem.166.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebuchi K., Wong G. G., Clark S. C., Ihle J. N., Hirai Y., Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9035–9039. doi: 10.1073/pnas.84.24.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler V., Thorens B., de Kossodo S., Allet B., Eliason J. F., Thatcher D., Farber N., Vassalli P. Stimulation of hematopoiesis in vivo by recombinant bacterial murine interleukin 3. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1001–1005. doi: 10.1073/pnas.83.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary A. G., Ikebuchi K., Hirai Y., Wong G. G., Yang Y. C., Clark S. C., Ogawa M. Synergism between interleukin-6 and interleukin-3 in supporting proliferation of human hematopoietic stem cells: comparison with interleukin-1 alpha. Blood. 1988 Jun;71(6):1759–1763. [PubMed] [Google Scholar]
- Lord B. I., Molineux G., Testa N. G., Kelly M., Spooncer E., Dexter T. M. The kinetic response of haemopoietic precursor cells, in vivo, to highly purified, recombinant interleukin-3. Lymphokine Res. 1986 Spring;5(2):97–104. [PubMed] [Google Scholar]
- Lotem J., Sachs L. In vivo control of differentiation of myeloid leukemic cells by recombinant granulocyte-macrophage colony-stimulating factor and interleukin 3. Blood. 1988 Feb;71(2):375–382. [PubMed] [Google Scholar]
- Metcalf D., Begley C. G., Johnson G. R., Nicola N. A., Lopez A. F., Williamson D. J. Effects of purified bacterially synthesized murine multi-CSF (IL-3) on hematopoiesis in normal adult mice. Blood. 1986 Jul;68(1):46–57. [PubMed] [Google Scholar]
- Muraguchi A., Hirano T., Tang B., Matsuda T., Horii Y., Nakajima K., Kishimoto T. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988 Feb 1;167(2):332–344. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prystowsky M. B., Otten G., Naujokas M. F., Vardiman J., Ihle J. N., Goldwasser E., Fitch F. W. Multiple hemopoietic lineages are found after stimulation of mouse bone marrow precursor cells with interleukin 3. Am J Pathol. 1984 Nov;117(2):171–179. [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., May L. T. Human interferon-beta 2. J Interferon Res. 1987 Oct;7(5):521–527. doi: 10.1089/jir.1987.7.521. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Peptide growth factors are multifunctional. Nature. 1988 Mar 17;332(6161):217–219. doi: 10.1038/332217a0. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., Keys M., Ni R. X., del Castillo J., Dakay E. B. The contributions of adrenal hormones, hemodynamic factors, and the endotoxin-related stress reaction to stable prostaglandin analog-induced peripheral lymphopenia and neutrophilia. J Leukoc Biol. 1988 Jan;43(1):5–10. doi: 10.1002/jlb.43.1.5. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., del Castillo J., Guo K. Z. In vivo hematologic effects of recombinant interleukin-6 on hematopoiesis and circulating numbers of RBCs and WBCs. Blood. 1989 Jan;73(1):108–110. [PubMed] [Google Scholar]
- Ulich T. R., del Castillo J., Guo K., Souza L. The hematologic effects of chronic administration of the monokines tumor necrosis factor, interleukin-1, and granulocyte-colony stimulating factor on bone marrow and circulation. Am J Pathol. 1989 Jan;134(1):149–159. [PMC free article] [PubMed] [Google Scholar]
- Ulich T. R., del Castillo J., Keys M., Granger G. A., Ni R. X. Kinetics and mechanisms of recombinant human interleukin 1 and tumor necrosis factor-alpha-induced changes in circulating numbers of neutrophils and lymphocytes. J Immunol. 1987 Nov 15;139(10):3406–3415. [PubMed] [Google Scholar]
- Ulich T. R., del Castillo J., Keys M., Granger G. A. Recombinant human alpha lymphotoxin (tumor necrosis factor-beta) induces peripheral neutrophilia and lymphopenia in the rat. Am J Pathol. 1987 Jul;128(1):5–12. [PMC free article] [PubMed] [Google Scholar]
- Ulich T. R., del Castillo J., Ni R. X., Bikhazi N., Calvin L. Mechanisms of tumor necrosis factor alpha-induced lymphopenia, neutropenia, and biphasic neutrophilia: a study of lymphocyte recirculation and hematologic interactions of TNF alpha with endogenous mediators of leukocyte trafficking. J Leukoc Biol. 1989 Feb;45(2):155–167. doi: 10.1002/jlb.45.2.155. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., del Castillo J., Souza L. Kinetics and mechanisms of recombinant human granulocyte-colony stimulating factor-induced neutrophilia. Am J Pathol. 1988 Dec;133(3):630–638. [PMC free article] [PubMed] [Google Scholar]