Abstract
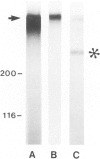
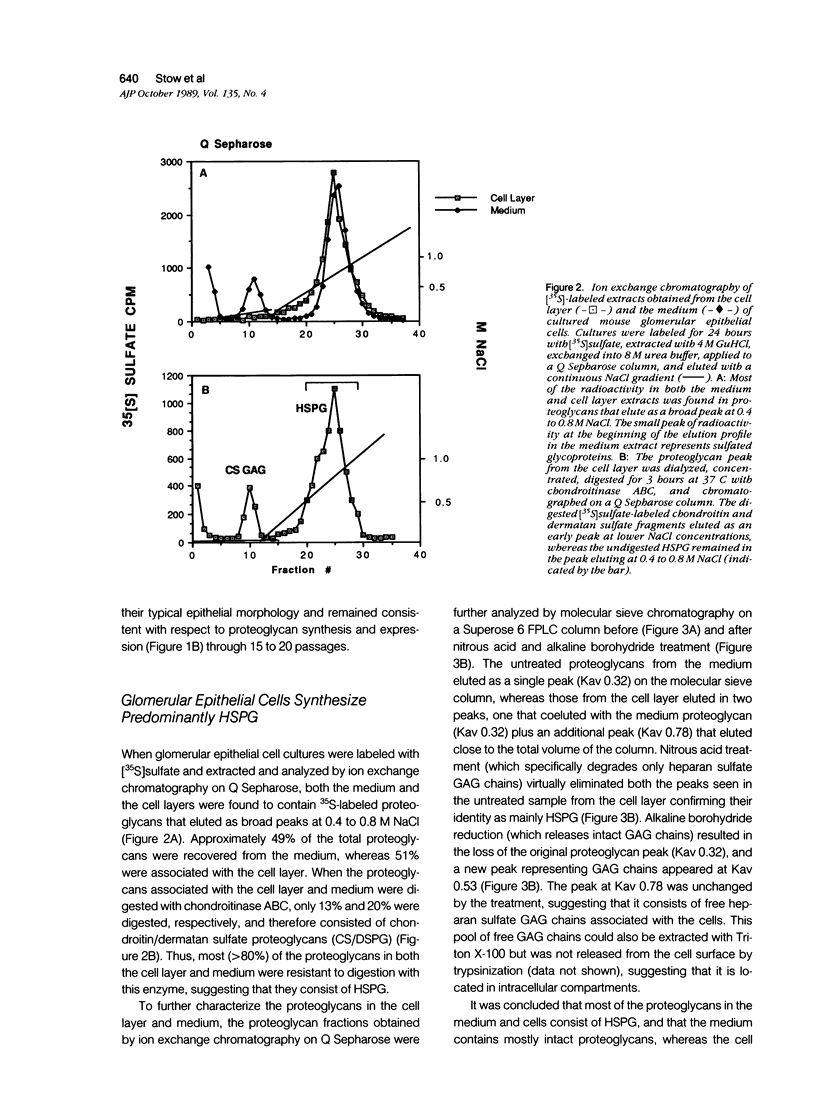
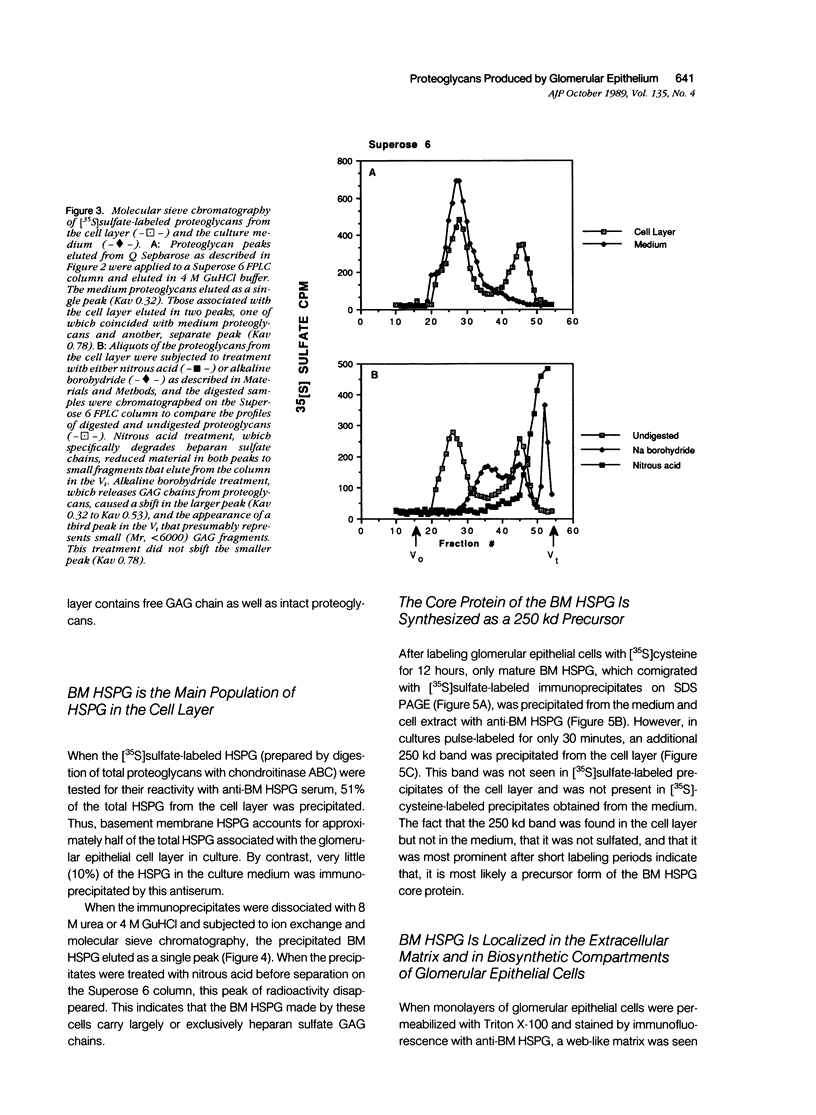
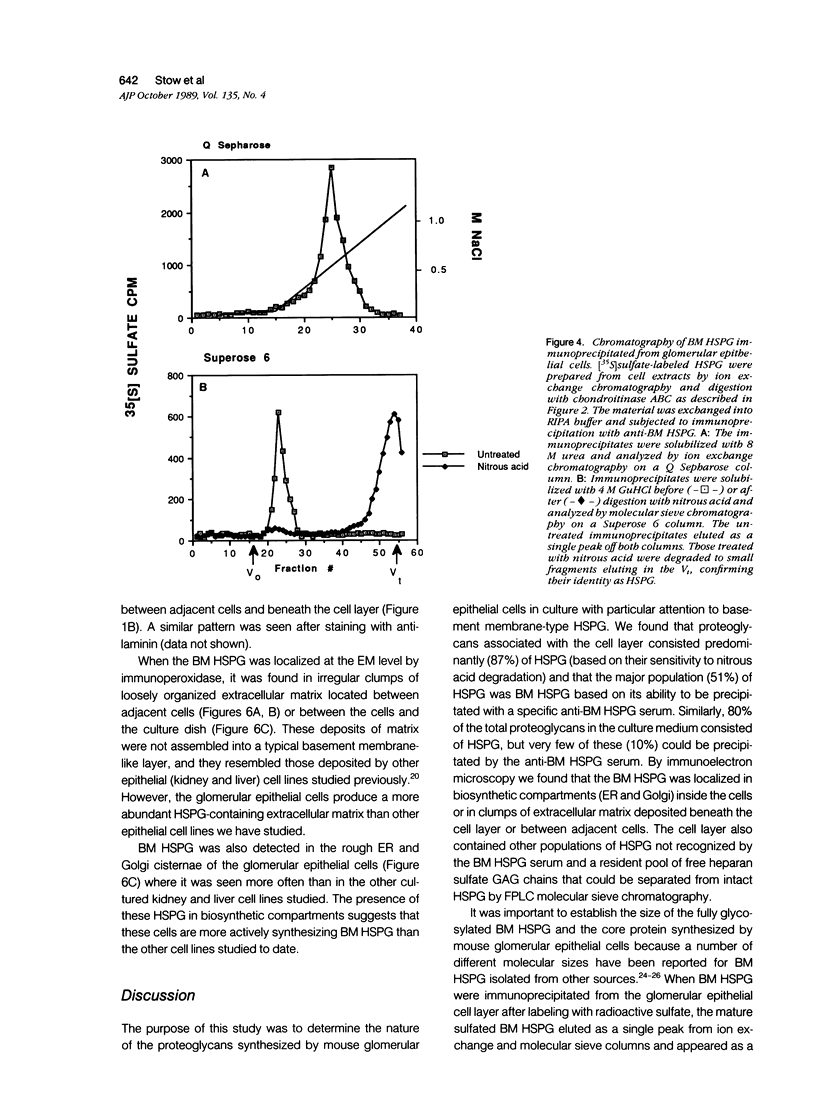
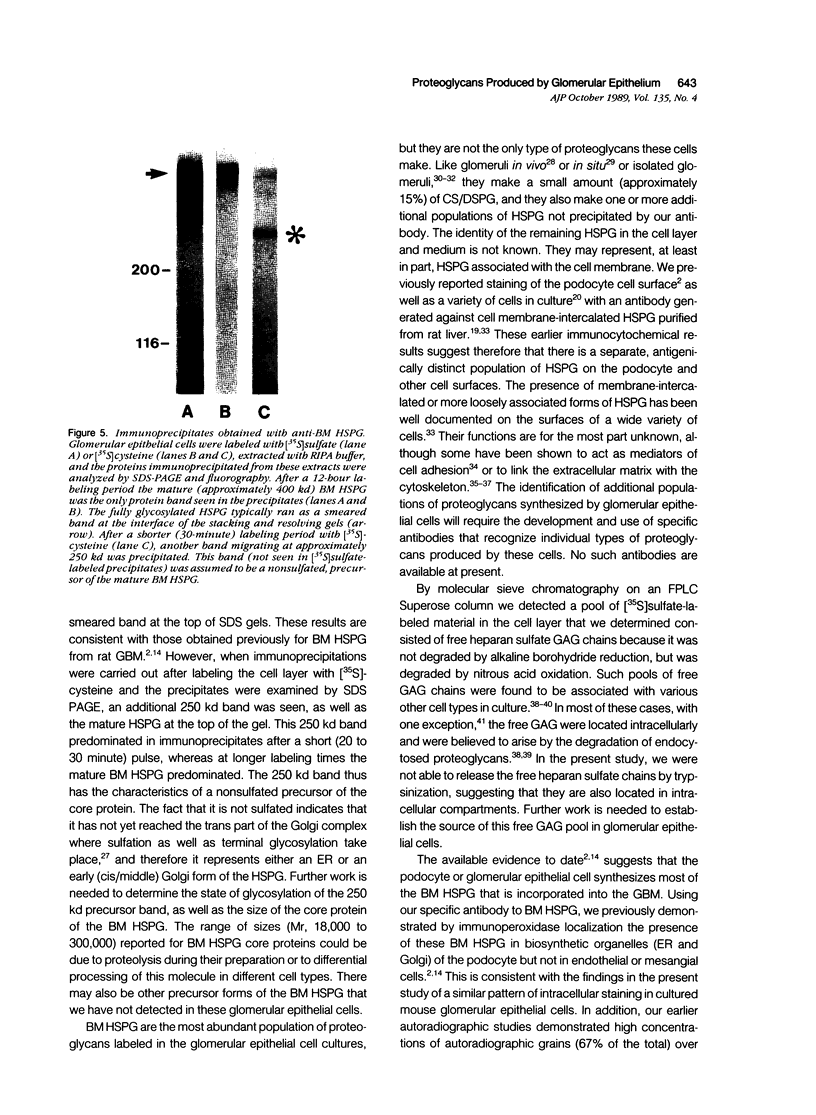
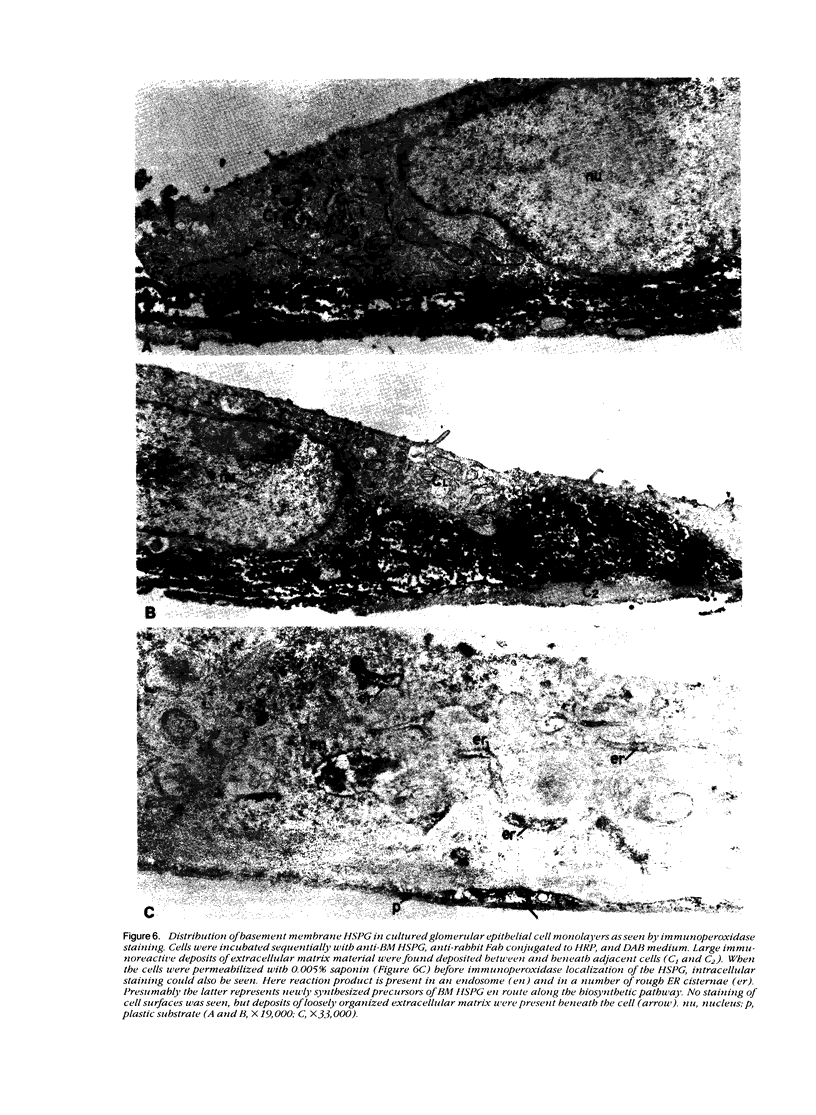
The production and distribution of basement membrane-type heparan sulfate proteoglycans (BM HSPG) were investigated in a mouse glomerular epithelial cell line. Confluent cell monolayers were radiolabeled with [35S]sulfate or [35S]cysteine. Proteoglycans were isolated from the medium and cell layers by ion exchange chromatography and their nature determined by enzyme digestion (chondroitinase ABC) or degradative treatment (nitrous acid). It was found that more than 80% of the proteoglycans in both the cell layer and medium were heparan sulfate proteoglycans (HSPG) based on their susceptibility to nitrous acid degradation. More than half of the HSPG in the cell layer could be precipitated with an antiserum that specifically recognizes BM HSPG; only 10% of those released into the medium were precipitated with this antiserum. When immunoprecipitates of [35S] sulfate-labeled proteoglycans were analyzed by SDS-PAGE, the mature proteoglycans ran as a broad band at the top of the gel. When immunoprecipitates of [35S]cysteine-labeled proteoglycans were similarly analyzed, a 250 kd precursor core protein band was seen in addition to the mature proteoglycan. When BM HSPG were localized by immunofluorescence and immunoelectron microscopy (immunoperoxidase), they were found intracellularly in biosynthetic compartments (ER and Golgi cisternae) and extracellularly in deposits of basement membrane-like matrix located beneath and between the cells. These results indicate that l) BM HSPG are the predominant type of proteoglycans made by glomerular epithelial cells in culture; 2) these HSPG are assembled into a loosely organized matrix that is deposited beneath and between the cells; and 3) this cell type produces a higher proportion of BM HSPG than other cultured epithelial cells studied previously.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienkowski M. J., Conrad H. E. Kinetics of proteoheparan sulfate synthesis, secretion, endocytosis, and catabolism by a hepatocyte cell line. J Biol Chem. 1984 Nov 10;259(21):12989–12996. [PubMed] [Google Scholar]
- Brauker J. H., Wang J. L. Nonlysosomal processing of cell-surface heparan sulfate proteoglycans. Studies of I-cells and NH4Cl-treated normal cells. J Biol Chem. 1987 Sep 25;262(27):13093–13101. [PubMed] [Google Scholar]
- Carey D. J., Todd M. S. A cytoskeleton-associated plasma membrane heparan sulfate proteoglycan in Schwann cells. J Biol Chem. 1986 Jun 5;261(16):7518–7525. [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Caulfield J. P., Farquhar M. G. Loss of anionic sites from the glomerular basement membrane in aminonucleoside nephrosis. Lab Invest. 1978 Nov;39(5):505–512. [PubMed] [Google Scholar]
- Cifonelli J. A., King J. The distribution of 2-acetamido-2-deoxy-D-glucose residues in mammalian heparins. Carbohydr Res. 1972 Feb;21(2):173–186. doi: 10.1016/s0008-6215(00)82144-8. [DOI] [PubMed] [Google Scholar]
- Cohen M. P., Surma M. L. [(35)S]sulfate incorporation into glomerular basement membrane glycosaminoglycans is decreased in experimental diabetes. J Lab Clin Med. 1981 Nov;98(5):715–722. [PubMed] [Google Scholar]
- Farquhar M. G. Progress in unraveling pathways of Golgi traffic. Annu Rev Cell Biol. 1985;1:447–488. doi: 10.1146/annurev.cb.01.110185.002311. [DOI] [PubMed] [Google Scholar]
- Foidart J. M., Foidart J. B., Mahieu P. R. Synthesis of collagen and fibronectin by glomerular cells in culture. Ren Physiol. 1980;3(1-6):183–192. doi: 10.1159/000172760. [DOI] [PubMed] [Google Scholar]
- Hayman E. G., Oldberg A., Martin G. R., Ruoslahti E. Codistribution of heparan sulfate proteoglycan, laminin, and fibronectin in the extracellular matrix of normal rat kidney cells and their coordinate absence in transformed cells. J Cell Biol. 1982 Jul;94(1):28–35. doi: 10.1083/jcb.94.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hök M., Kjellén L., Johansson S. Cell-surface glycosaminoglycans. Annu Rev Biochem. 1984;53:847–869. doi: 10.1146/annurev.bi.53.070184.004215. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S. Biophysiology of glomerular filtration and proteinuria. Lab Invest. 1984 Jul;51(1):7–21. [PubMed] [Google Scholar]
- Kanwar Y. S., Hascall V. C., Farquhar M. G. Partial characterization of newly synthesized proteoglycans isolated from the glomerular basement membrane. J Cell Biol. 1981 Aug;90(2):527–532. doi: 10.1083/jcb.90.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Rosenzweig L. J., Jakubowski M. L. Distribution of de novo synthesized sulfated glycosaminoglycans in the glomerular basement membrane and mesangial matrix. Lab Invest. 1983 Aug;49(2):216–225. [PubMed] [Google Scholar]
- Kanwar Y. S., Rosenzweig L. J., Linker A., Jakubowski M. L. Decreased de novo synthesis of glomerular proteoglycans in diabetes: biochemical and autoradiographic evidence. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2272–2275. doi: 10.1073/pnas.80.8.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Veis A., Kimura J. H., Jakubowski M. L. Characterization of heparan sulfate-proteoglycan of glomerular basement membranes. Proc Natl Acad Sci U S A. 1984 Feb;81(3):762–766. doi: 10.1073/pnas.81.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Koike Y., Ito Y., Suzuki S., Kimata K. Multiple forms of heparan sulfate proteoglycans in the Engelbreth-Holm-Swarm mouse tumor. The occurrence of high density forms bearing both heparan sulfate and chondroitin sulfate side chains. J Biol Chem. 1987 May 25;262(15):7180–7188. [PubMed] [Google Scholar]
- Klein D. J., Brown D. M., Oegema T. R., Brenchley P. E., Anderson J. C., Dickinson M. A., Horigan E. A., Hassell J. R. Glomerular basement membrane proteoglycans are derived from a large precursor. J Cell Biol. 1988 Mar;106(3):963–970. doi: 10.1083/jcb.106.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D. J., Brown D. M., Oegema T. R., Jr Partial characterization of heparan and dermatan sulfate proteoglycans synthesized by normal rat glomeruli. J Biol Chem. 1986 Dec 15;261(35):16636–16652. [PubMed] [Google Scholar]
- Klein D. J., Dehnel P. J., Oegema T. R., Brown D. M. Alterations in proteoglycan metabolism in the nephrotic syndrome induced by the aminonucleoside of puromycin. Lab Invest. 1984 May;50(5):543–551. [PubMed] [Google Scholar]
- Kreisberg J. I., Karnovsky M. J. Glomerular cells in culture. Kidney Int. 1983 Mar;23(3):439–447. doi: 10.1038/ki.1983.40. [DOI] [PubMed] [Google Scholar]
- Lark M. W., Culp L. A. Multiple classes of heparan sulfate proteoglycans from fibroblast substratum adhesion sites. Affinity fractionation on columns of platelet factor 4, plasma fibronectin, and octyl-sepharose. J Biol Chem. 1984 Jun 10;259(11):6773–6782. [PubMed] [Google Scholar]
- Lemkin M. C., Farquhar M. G. Sulfated and nonsulfated glycosaminoglycans and glycopeptides are synthesized by kidney in vivo and incorporated into glomerular basement membranes. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1726–1730. doi: 10.1073/pnas.78.3.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay K., Striker L. J., Elliot S., Pinkert C. A., Brinster R. L., Striker G. E. Glomerular epithelial, mesangial, and endothelial cell lines from transgenic mice. Kidney Int. 1988 Mar;33(3):677–684. doi: 10.1038/ki.1988.53. [DOI] [PubMed] [Google Scholar]
- Melnick G. F., Ladoulis C. T., Cavallo T. Decreased anionic groups and increased permeability precedes deposition of immune complexes in the glomerular capillary wall. Am J Pathol. 1981 Nov;105(2):114–120. [PMC free article] [PubMed] [Google Scholar]
- Mynderse L. A., Hassell J. R., Kleinman H. K., Martin G. R., Martinez-Hernandez A. Loss of heparan sulfate proteoglycan from glomerular basement membrane of nephrotic rats. Lab Invest. 1983 Mar;48(3):292–302. [PubMed] [Google Scholar]
- Oohira A., Wight T. N., Bornstein P. Sulfated proteoglycans synthesized by vascular endothelial cells in culture. J Biol Chem. 1983 Feb 10;258(3):2014–2021. [PubMed] [Google Scholar]
- Piepkorn M., Hovingh P., Linker A. Evidence for independent metabolism and cell surface localization of cell surface localization of cellular proteoglycans and glycosaminoglycan free chains. J Cell Physiol. 1988 May;135(2):189–199. doi: 10.1002/jcp.1041350206. [DOI] [PubMed] [Google Scholar]
- Rapraeger A., Jalkanen M., Bernfield M. Cell surface proteoglycan associates with the cytoskeleton at the basolateral cell surface of mouse mammary epithelial cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2683–2696. doi: 10.1083/jcb.103.6.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach D. H., Wagner C. W., Star V. L., Martin G. R., Brown K. S., Yoon J. W. Reduced synthesis of basement membrane heparan sulfate proteoglycan in streptozotocin-induced diabetic mice. J Biol Chem. 1983 Oct 10;258(19):11672–11677. [PubMed] [Google Scholar]
- Schneeberger E. E., Stavrakis G., McCarthy K. Alterations in glomerular anionic sites in autologous immune complex nephritis. Lab Invest. 1983 Oct;49(4):445–452. [PubMed] [Google Scholar]
- Shimomura H., Spiro R. G. Studies on macromolecular components of human glomerular basement membrane and alterations in diabetes. Decreased levels of heparan sulfate proteoglycan and laminin. Diabetes. 1987 Mar;36(3):374–381. doi: 10.2337/diab.36.3.374. [DOI] [PubMed] [Google Scholar]
- Stow J. L., Farquhar M. G. Distinctive populations of basement membrane and cell membrane heparan sulfate proteoglycans are produced by cultured cell lines. J Cell Biol. 1987 Jul;105(1):529–539. doi: 10.1083/jcb.105.1.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow J. L., Glasgow E. F., Handley C. J., Hascall V. C. Biosynthesis of proteoglycans by isolated rabbit glomeruli. Arch Biochem Biophys. 1983 Sep;225(2):950–957. doi: 10.1016/0003-9861(83)90110-8. [DOI] [PubMed] [Google Scholar]
- Stow J. L., Kjéllen L., Unger E., Hök M., Farquhar M. G. Heparan sulfate proteoglycans are concentrated on the sinusoidal plasmalemmal domain and in intracellular organelles of hepatocytes. J Cell Biol. 1985 Mar;100(3):975–980. doi: 10.1083/jcb.100.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow J. L., Sawada H., Farquhar M. G. Basement membrane heparan sulfate proteoglycans are concentrated in the laminae rarae and in podocytes of the rat renal glomerulus. Proc Natl Acad Sci U S A. 1985 May;82(10):3296–3300. doi: 10.1073/pnas.82.10.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striker G. E., Killen P. D., Farin F. M. Human glomerular cells in vitro: isolation and characterization. Transplant Proc. 1980 Sep;12(3 Suppl 1):88–99. [PubMed] [Google Scholar]
- Striker G. E., Striker L. J. Glomerular cell culture. Lab Invest. 1985 Aug;53(2):122–131. [PubMed] [Google Scholar]
- Vernier R. L., Klein D. J., Sisson S. P., Mahan J. D., Oegema T. R., Brown D. M. Heparan sulfate--rich anionic sites in the human glomerular basement membrane. Decreased concentration in congenital nephrotic syndrome. N Engl J Med. 1983 Oct 27;309(17):1001–1009. doi: 10.1056/NEJM198310273091701. [DOI] [PubMed] [Google Scholar]
- Woods A., Hök M., Kjellén L., Smith C. G., Rees D. A. Relationship of heparan sulfate proteoglycans to the cytoskeleton and extracellular matrix of cultured fibroblasts. J Cell Biol. 1984 Nov;99(5):1743–1753. doi: 10.1083/jcb.99.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagishita M., Hascall V. C. Metabolism of proteoglycans in rat ovarian granulosa cell culture. Multiple intracellular degradative pathways and the effect of chloroquine. J Biol Chem. 1984 Aug 25;259(16):10270–10283. [PubMed] [Google Scholar]