Abstract
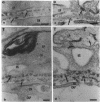
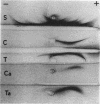
Gentamicin during gestation alters glomerular basement membrane development. A drug-induced nephrotoxicity was described for neonates after gentamicin was given intraperitoneally to pregnant Wistar rats; glomerular alterations and changes in permselectivity were important. We investigated the ultrastructure of the glomerular basement membrane (GBM), the arrangement of anionic sites, and the urinary proteins at two ages, with 1-day- and 12-month-old control and prenatally exposed animals. For neonates, the pattern of glomerular differentiation was similar, anionic sites were made of heparan sulfate proteoglycans, and the GBM had the same total thickness in both groups. After transplacental gentamicin exposure, the lamina densa was larger; the laminae rarae were thinner; the density of anionic sites was increased; the levels of hydroxyproline, sulfate, and hexuronic acid in the kidney were increased; and the immunoelectrophoresis of urinary proteins was abnormal. For adults, prenatal exposure to gentamicin led to altered juxta-medullary glomeruli with a larger GBM and abundant anionic sites, especially in the lamina densa, and to a protein excretion different from that of controls. Thus, gentamicin administered during pregnancy leads to permanent alterations of the GBM with modifications of both the layers and the anionic sites, possibly because of a perturbed protein metabolism. These altered glomeruli are at risk during life and could be the starting point for a kidney disease.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bakala H., Cornet S., Cheignon M., Djaziri R., Schaeverbeke J. Basement membrane proteoglycans and anionic sites in the fetal rat kidney during late gestation. J Morphol. 1988 Apr;196(1):1–14. doi: 10.1002/jmor.1051960102. [DOI] [PubMed] [Google Scholar]
- Bakala H., Geloso-Meyer A., Cheignon M., Schaeverbeke J. Differentiation of the glomerular filtration barrier in the rat fetus: possible role of collagen. Connect Tissue Res. 1985;13(4):283–290. doi: 10.3109/03008208509152409. [DOI] [PubMed] [Google Scholar]
- Bard J. B., Woolf A. S. Nephrogenesis and the development of renal disease. Nephrol Dial Transplant. 1992;7(7):563–572. doi: 10.1093/ndt/7.7.563. [DOI] [PubMed] [Google Scholar]
- Barnes J. L., Radnik R. A., Gilchrist E. P., Venkatachalam M. A. Size and charge selective permeability defects induced in glomerular basement membrane by a polycation. Kidney Int. 1984 Jan;25(1):11–19. doi: 10.1038/ki.1984.2. [DOI] [PubMed] [Google Scholar]
- Baylis C. Gentamicin-induced glomerulotoxicity in the pregnant rat. Am J Kidney Dis. 1989 Feb;13(2):108–113. doi: 10.1016/s0272-6386(89)80127-1. [DOI] [PubMed] [Google Scholar]
- Baylis C., Rennke H. R., Brenner B. M. Mechanisms of the defect in glomerular ultrafiltration associated with gentamicin administration. Kidney Int. 1977 Nov;12(5):344–353. doi: 10.1038/ki.1977.121. [DOI] [PubMed] [Google Scholar]
- Bennett C. M., Glassock R. J., Chang R. L., Deen W. M., Robertson C. R., Brenner B. M., Troy J. L., ueki I. R., Rasmussen B. Permselectivity of the glomerular capillary wall. Studies of experimental glomerulonephritis in the rat using dextran sulfate. J Clin Invest. 1976 May;57(5):1287–1294. doi: 10.1172/JCI108396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett W. M. Aminoglycoside nephrotoxicity. Nephron. 1983;35(2):73–77. doi: 10.1159/000183050. [DOI] [PubMed] [Google Scholar]
- Bergman I., Loxley R. The determination of hydroxyproline in urine hydrolysates. Clin Chim Acta. 1970 Feb;27(2):347–349. doi: 10.1016/0009-8981(70)90355-4. [DOI] [PubMed] [Google Scholar]
- Blau E. B., Haas J. E. Glomerular sialic acid and proteinuria in human renal disease. Lab Invest. 1973 Apr;28(4):477–481. [PubMed] [Google Scholar]
- Caulfield J. P., Farquhar M. G. Distribution of annionic sites in glomerular basement membranes: their possible role in filtration and attachment. Proc Natl Acad Sci U S A. 1976 May;73(5):1646–1650. doi: 10.1073/pnas.73.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Farquhar M. G. The permeability of glomerular capillaries to graded dextrans. Identification of the basement membrane as the primary filtration barrier. J Cell Biol. 1974 Dec;63(3):883–903. doi: 10.1083/jcb.63.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. L., Deen W. M., Robertson C. R., Brenner B. M. Permselectivity of the glomerular capillary wall: III. Restricted transport of polyanions. Kidney Int. 1975 Oct;8(4):212–218. doi: 10.1038/ki.1975.104. [DOI] [PubMed] [Google Scholar]
- Chang R. L., Ueki I. F., Troy J. L., Deen W. M., Robertson C. R., Brenner B. M. Permselectivity of the glomerular capillary wall to macromolecules. II. Experimental studies in rats using neutral dextran. Biophys J. 1975 Sep;15(9):887–906. doi: 10.1016/S0006-3495(75)85863-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan R. H., Jukkola A. F., Arant B. S., Jr Pathophysiologic evidence of gentamicin nephrotoxicity in neonatal puppies. Pediatr Res. 1980 Nov;14(11):1204–1211. doi: 10.1203/00006450-198011000-00011. [DOI] [PubMed] [Google Scholar]
- Gilbert T., Lelievre-Pegorier M., Malienou R., Meulemans A., Merlet-Benichou C. Effects of prenatal and postnatal exposure to gentamicin on renal differentiation in the rat. Toxicology. 1987 Mar;43(3):301–313. doi: 10.1016/0300-483x(87)90089-8. [DOI] [PubMed] [Google Scholar]
- Gilbert T., Nabarra B., Merlet-Bénichou C. Light- and electron-microscopic analysis of the kidney in newborn rats exposed to gentamicin in utero. Am J Pathol. 1988 Jan;130(1):33–43. [PMC free article] [PubMed] [Google Scholar]
- Houghton D. C., Hartnett M., Campbell-Boswell M., Porter G., Bennett W. A light and electron microscopic analysis of gentamicin nephrotoxicity in rats. Am J Pathol. 1976 Mar;82(3):589–612. [PMC free article] [PubMed] [Google Scholar]
- Kaloyanides G. J., Pastoriza-Munoz E. Aminoglycoside nephrotoxicity. Kidney Int. 1980 Nov;18(5):571–582. doi: 10.1038/ki.1980.175. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Anionic sites in the glomerular basement membrane. In vivo and in vitro localization to the laminae rarae by cationic probes. J Cell Biol. 1979 Apr;81(1):137–153. doi: 10.1083/jcb.81.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1303–1307. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Linker A., Farquhar M. G. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol. 1980 Aug;86(2):688–693. doi: 10.1083/jcb.86.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Luft F. C., Bloch R., Sloan R. S., Yum M. N., Costello R., Maxwell D. R. Comparative nephrotoxicity of aminoglycoside antibiotics in rats. J Infect Dis. 1978 Oct;138(4):541–545. doi: 10.1093/infdis/138.4.541. [DOI] [PubMed] [Google Scholar]
- Mallie J. P., Gerard H., Gerard A. Gentamicin administration to pregnant rats: effect on fetal renal development in utero. Dev Pharmacol Ther. 1984;7 (Suppl 1):89–92. doi: 10.1159/000457234. [DOI] [PubMed] [Google Scholar]
- Mallié J. P., Coulon G., Billerey C., Faucourt A., Morin J. P. In utero aminoglycosides-induced nephrotoxicity in rat neonates. Kidney Int. 1988 Jan;33(1):36–44. doi: 10.1038/ki.1988.6. [DOI] [PubMed] [Google Scholar]
- Mallié J. P., Gerard H., Gerard A. In-utero gentamicin-induced nephrotoxicity in rats. Pediatr Pharmacol (New York) 1986;5(4):229–239. [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Michael A. F., Blau E., Vernier R. L. Glomerular polyanion. Alteration in aminonucleoside nephrosis. Lab Invest. 1970 Dec;23(6):649–657. [PubMed] [Google Scholar]
- Mynderse L. A., Hassell J. R., Kleinman H. K., Martin G. R., Martinez-Hernandez A. Loss of heparan sulfate proteoglycan from glomerular basement membrane of nephrotic rats. Lab Invest. 1983 Mar;48(3):292–302. [PubMed] [Google Scholar]
- Olbricht C. J., Fink M., Gutjahr E. Alterations in lysosomal enzymes of the proximal tubule in gentamicin nephrotoxicity. Kidney Int. 1991 Apr;39(4):639–646. doi: 10.1038/ki.1991.76. [DOI] [PubMed] [Google Scholar]
- Rajchgot P., Prober C. G., Soldin S., Perlman M., Good F., Harding E., Klein J., MacLeod S. Aminoglycoside-related nephrotoxicity in the premature newborn. Clin Pharmacol Ther. 1984 Mar;35(3):394–401. doi: 10.1038/clpt.1984.49. [DOI] [PubMed] [Google Scholar]
- Reeves W. H., Kanwar Y. S., Farquhar M. G. Assembly of the glomerular filtration surface. Differentiation of anionic sites in glomerular capillaries of newborn rat kidney. J Cell Biol. 1980 Jun;85(3):735–753. doi: 10.1083/jcb.85.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennke H. G., Patel Y., Venkatachalam M. A. Glomerular filtration of proteins: clearance of anionic, neutral, and cationic horseradish peroxidase in the rat. Kidney Int. 1978 Apr;13(4):278–288. doi: 10.1038/ki.1978.41. [DOI] [PubMed] [Google Scholar]
- Schaeverbeke J., Cheignon M. Differentiation of glomerular filter and tubular reabsorption apparatus during foetal development of the rat kidney. J Embryol Exp Morphol. 1980 Aug;58:157–175. [PubMed] [Google Scholar]
- Schurer J. W., Kalicharan D., Hoedemaeker P. J., Molenaar I. The use of polyethyleneimine for demonstration of anionic sites in basement membranes and collagen fibrils. J Histochem Cytochem. 1978 Aug;26(8):688–689. doi: 10.1177/26.8.690407. [DOI] [PubMed] [Google Scholar]
- Smaoui H., Mallie J. P., Cheignon M., Borot C., Schaeverbeke J. Glomerular alterations in rat neonates after transplacental exposure to gentamicin. Nephron. 1991;59(4):626–631. doi: 10.1159/000186655. [DOI] [PubMed] [Google Scholar]
- Solez K., Racusen L. C., Olsen S. The pathology of drug nephrotoxicity. J Clin Pharmacol. 1983 Oct;23(10):484–490. doi: 10.1002/j.1552-4604.1983.tb01794.x. [DOI] [PubMed] [Google Scholar]
- Sorokin L., Ekblom P. Development of tubular and glomerular cells of the kidney. Kidney Int. 1992 Mar;41(3):657–664. doi: 10.1038/ki.1992.101. [DOI] [PubMed] [Google Scholar]
- Terho T. T., Hartiala K. Method for determination of the sulfate content of glycosaminoglycans. Anal Biochem. 1971 Jun;41(2):471–476. doi: 10.1016/0003-2697(71)90167-9. [DOI] [PubMed] [Google Scholar]
- Timpl R. Recent advances in the biochemistry of glomerular basement membrane. Kidney Int. 1986 Sep;30(3):293–298. doi: 10.1038/ki.1986.183. [DOI] [PubMed] [Google Scholar]
- Vernier R. L., Klein D. J., Sisson S. P., Mahan J. D., Oegema T. R., Brown D. M. Heparan sulfate--rich anionic sites in the human glomerular basement membrane. Decreased concentration in congenital nephrotic syndrome. N Engl J Med. 1983 Oct 27;309(17):1001–1009. doi: 10.1056/NEJM198310273091701. [DOI] [PubMed] [Google Scholar]
- Weber M. Basement membrane proteins. Kidney Int. 1992 Mar;41(3):620–628. doi: 10.1038/ki.1992.95. [DOI] [PubMed] [Google Scholar]