Abstract
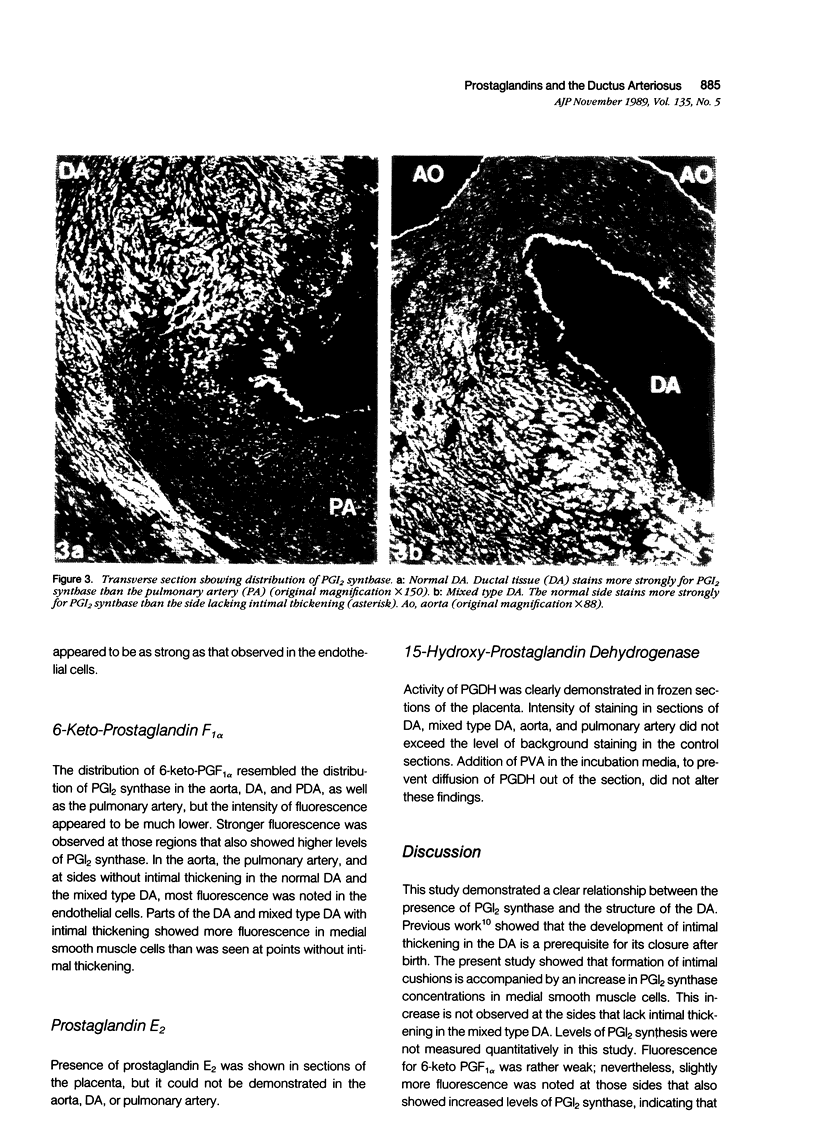
The presence of prostacyclin synthase (PGI2 synthase), 6-keto-prostaglandin F1 alpha (6k-PGF1 alpha), and the stable hydrolysis product of prostacyclin (PGI2), prostaglandin E2 (PGE2), as well as the activity of 15-hydroxy-prostaglandin dehydrogenase (PGDH) were studied in the aorta, pulmonary artery, the normal ductus arteriosus (DA), and persistent DA (PDA) of the dog using histochemical and immunohistochemical techniques. The normal DA is characterized by the development of intimal thickening, a process that does not occur in the persistent DA. Distribution of PGI2 synthase was identical in the aorta, pulmonary artery, and persistent DA. In these vessels endothelial cells contained higher levels of PGI2 synthase as compared with medial smooth muscle cells. In the normal DA, levels of PGI2 synthase were clearly higher in smooth muscle cells at the sites of intimal thickening than at other sites. Distribution of 6-keto-PGF1 alpha resembled the localization of PGI2 synthase. Presence of PGE2 and activity of PGDH could not be demonstrated. The results demonstrated existence of a clear relationship between ductal morphology and the presence of PGI2 synthase. This finding suggests a more important role for PGI2 in regulating ductal patency than has heretofore been appreciated. It was assumed that the role of PGI2 in regulating ductal patency is, at birth, at least overruled by the constrictive effect of the cytochrome P450 mono-oxygenase mechanism. It is still possible to attribute a role to PGI2 in the regulation of cushion formation. Once smooth muscle cell activity has been enhanced by the presence of a glycosaminoglycan rich environment, increase in PGI2 may produce a concurrent inhibition of smooth muscle cell growth.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bocquet J., Langris M., Daireaux M., Jouis V., Pujol J. P., Beliard R., Loyau G. Mononuclear cell-mediated modulation of synovial cell metabolism. II. Increased hyaluronic acid synthesis by a monocyte cell factor (MCF). Exp Cell Res. 1985 Sep;160(1):9–18. doi: 10.1016/0014-4827(85)90231-9. [DOI] [PubMed] [Google Scholar]
- Clyman R. I. Ductus arteriosus: current theories of prenatal and postnatal regulation. Semin Perinatol. 1987 Jan;11(1):64–71. [PubMed] [Google Scholar]
- Clyman R. I., Mauray F., Roman C., Rudolph A. M. PGE2 is a more potent vasodilator of the lamb ductus arteriosus than is either PGI2 or 6 keto PGF1alpha. Prostaglandins. 1978 Aug;16(2):259–264. doi: 10.1016/0090-6980(78)90028-x. [DOI] [PubMed] [Google Scholar]
- Coceani F., Bodach E., White E., Bishai I., Olley P. M. Prostaglandin I2 is less relaxant than prostaglandin E2 on the lamb ductus arteriosus. Prostaglandins. 1978 Apr;15(4):551–556. doi: 10.1016/0090-6980(78)90051-5. [DOI] [PubMed] [Google Scholar]
- Coceani F., Breen C. A., Lees J. G., Falck J. R., Olley P. M. Further evidence implicating a cytochrome P-450-mediated reaction in the contractile tension of the lamb ductus arteriosus. Circ Res. 1988 Mar;62(3):471–477. doi: 10.1161/01.res.62.3.471. [DOI] [PubMed] [Google Scholar]
- Coceani F., Huhtanen D., Hamilton N. C., Bishai I., Olley P. M. Involvement of intramural prostaglandin E2 in prenatal patency of the lamb ductus arteriosus. Can J Physiol Pharmacol. 1986 Jun;64(6):737–744. doi: 10.1139/y86-124. [DOI] [PubMed] [Google Scholar]
- De Reeder E. G., Girard N., Poelmann R. E., Van Munsteren J. C., Patterson D. F., Gittenberger-De Groot A. C. Hyaluronic acid accumulation and endothelial cell detachment in intimal thickening of the vessel wall. The normal and genetically defective ductus arteriosus. Am J Pathol. 1988 Sep;132(3):574–585. [PMC free article] [PubMed] [Google Scholar]
- Funk C. D., Powell W. S. Release of prostaglandins and monohydroxy and trihydroxy metabolites of linoleic and arachidonic acids by adult and fetal aortae and ductus arteriosus. J Biol Chem. 1985 Jun 25;260(12):7481–7488. [PubMed] [Google Scholar]
- Gittenberger-de Groot A. C. Persistent ductus arteriosus: most probably a primary congenital malformation. Br Heart J. 1977 Jun;39(6):610–618. doi: 10.1136/hrt.39.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittenberger-de Groot A. C., Strengers J. L., Mentink M., Poelmann R. E., Patterson D. F. Histologic studies on normal and persistent ductus arteriosus in the dog. J Am Coll Cardiol. 1985 Aug;6(2):394–404. doi: 10.1016/s0735-1097(85)80178-9. [DOI] [PubMed] [Google Scholar]
- Gittenberger-de Groot A. C., van Ertbruggen I., Moulaert A. J., Harinck E. The ductus arteriosus in the preterm infant: histologic and clinical observations. J Pediatr. 1980 Jan;96(1):88–93. doi: 10.1016/s0022-3476(80)80337-4. [DOI] [PubMed] [Google Scholar]
- Moonen P., Klok G., Keirse M. J. Immunohistochemical localisation of prostaglandin endoperoxide synthase and prostacyclin synthase in pregnant human myometrium. Eur J Obstet Gynecol Reprod Biol. 1985 Mar;19(3):151–158. doi: 10.1016/0028-2243(85)90149-2. [DOI] [PubMed] [Google Scholar]
- Nissen H. M., Andersen H. On the activity of prostaglandin-dehydrogenase system in the kidney. A histochemical study during hydration-dehydration and salt-repletion-salt-depletion. Histochemie. 1969;17(3):241–247. doi: 10.1007/BF00309868. [DOI] [PubMed] [Google Scholar]
- Olley P. M., Coceani F. Lipid mediators in the control of the ductus arteriosus. Am Rev Respir Dis. 1987 Jul;136(1):218–219. doi: 10.1164/ajrccm/136.1.218. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak C. R., Rangaraj G. Prostaglandin biosynthesis and catabolism in the lamb ductus arteriosus, aorta and pulmonary artery. Biochim Biophys Acta. 1978 Apr 28;529(1):13–20. doi: 10.1016/0005-2760(78)90098-x. [DOI] [PubMed] [Google Scholar]
- Patterson D. F. Epidemiologic and genetic studies of congenital heart disease in the dog. Circ Res. 1968 Aug;23(2):171–202. doi: 10.1161/01.res.23.2.171. [DOI] [PubMed] [Google Scholar]
- Patterson D. F., Pyle R. L., Buchanan J. W., Trautvetter E., Abt D. A. Hereditary patent ductus arteriosus and its sequelae in the dog. Circ Res. 1971 Jul;29(1):1–13. doi: 10.1161/01.res.29.1.1. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M. Prostaglandins and structural changes in pulmonary arteries. Am Rev Respir Dis. 1987 Sep;136(3):777–779. doi: 10.1164/ajrccm/136.3.777. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Skidgel R. A., Friedman W. F., Printz M. P. Prostaglandin biosynthetic activities of isolated fetal lamb ductus arteriosus, other blood vessels, and lung tissue. Pediatr Res. 1984 Jan;18(1):12–18. [PubMed] [Google Scholar]
- Smith W. L., DeWitt D. L., Allen M. L. Bimodal distribution of the prostaglandin I2 synthase antigen in smooth muscle cells. J Biol Chem. 1983 May 10;258(9):5922–5926. [PubMed] [Google Scholar]