Abstract
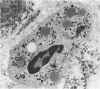
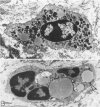
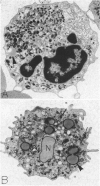
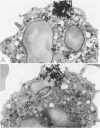
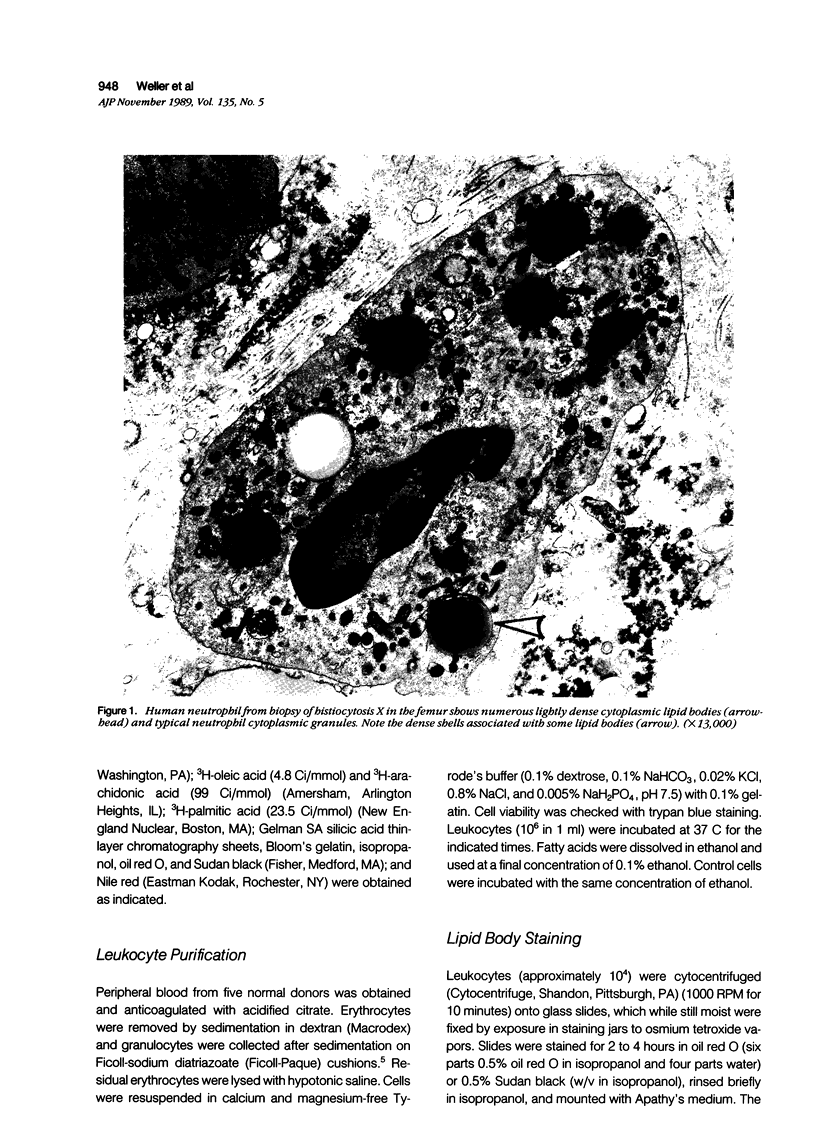
The morphology and function of cytoplasmic lipid bodies in human neutrophils were evaluated. By transmission electron microscopy, neutrophil lipid bodies were cytoplasmic inclusions, usually several microns in diameter, that occasionally coalesced to attain a diameter up to 7 microM. Neutrophil lipid bodies were not enveloped by membrane but were often surrounded by a more electron-dense shell at their periphery. Normal peripheral blood neutrophils contained an average of approximately one lipid body per cell. Lipid bodies appeared in greater numbers in neutrophils from inflammatory lesions. Perturbation of neutrophils during conventional methods of cell isolation and purification modestly increased lipid body numbers in neutrophils, whereas incubation of neutrophils with 1 microM oleic acid rapidly induced lipid body formation over 30 to 60 minutes. After granulocytes were incubated for 2 hours with 3H-fatty acids, including arachidonic, oleic, and palmitic acids, electron microscopic autoradiography demonstrated that lipid bodies represented the predominant intracellular sites of localization of each of the three 3H-fatty acids. There was lesser labeling noted in the perinuclear cisterna, but not in cell membranes. Virtually all of each of the three 3H-fatty acids incorporated by the neutrophils were esterified into chromatographically resolved classes of neutral lipids or phospholipids. These findings indicate that cytoplasmic lipid bodies are more prominent in neutrophils in vivo engaged in inflammatory responses and that these organelles in human neutrophils function as sites of deposition of esterified, incorporated fatty acids.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Burns C. P., Welshman I. R., Spector A. A. Differences in free fatty acid and glucose metabolism of human blood neutrophils and lymphocytes. Blood. 1976 Mar;47(3):431–437. [PubMed] [Google Scholar]
- Chilton F. H., Murphy R. C. Remodeling of arachidonate-containing phosphoglycerides within the human neutrophil. J Biol Chem. 1986 Jun 15;261(17):7771–7777. [PubMed] [Google Scholar]
- Coimbra A., Lopes-Vaz A. The presence of lipid droplets and the absence of stable sudanophilia in osmium-fixed human leukocytes. J Histochem Cytochem. 1971 Sep;19(9):551–557. doi: 10.1177/19.9.551. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., Dvorak H. F., Peters S. P., Shulman E. S., MacGlashan D. W., Jr, Pyne K., Harvey V. S., Galli S. J., Lichtenstein L. M. Lipid bodies: cytoplasmic organelles important to arachidonate metabolism in macrophages and mast cells. J Immunol. 1983 Dec;131(6):2965–2976. [PubMed] [Google Scholar]
- Dvorak A. M., Hammel I., Schulman E. S., Peters S. P., MacGlashan D. W., Jr, Schleimer R. P., Newball H. H., Pyne K., Dvorak H. F., Lichtenstein L. M. Differences in the behavior of cytoplasmic granules and lipid bodies during human lung mast cell degranulation. J Cell Biol. 1984 Nov;99(5):1678–1687. doi: 10.1083/jcb.99.5.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak A. M., Hammond M. E., Morgan E. S., Dvorak H. F. Ultrastructural studies of macrophages: in vitro removal of cell coat with macrophage inhibition factor (MIF)-containing lymphocyte culture supernatants; chloroform extraction, phospholipase digestion, and autoradiographic studies. J Reticuloendothel Soc. 1980 Feb;27(2):119–142. [PubMed] [Google Scholar]
- Fowler S. D., Greenspan P. Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: comparison with oil red O. J Histochem Cytochem. 1985 Aug;33(8):833–836. doi: 10.1177/33.8.4020099. [DOI] [PubMed] [Google Scholar]
- Greenspan P., Fowler S. D. Spectrofluorometric studies of the lipid probe, nile red. J Lipid Res. 1985 Jul;26(7):781–789. [PubMed] [Google Scholar]
- Greenspan P., Mayer E. P., Fowler S. D. Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 1985 Mar;100(3):965–973. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley H. P., Gordon G. B. The effects of long chain free fatty acids on human neutrophil function and structure. Lab Invest. 1976 Feb;34(2):216–222. [PubMed] [Google Scholar]
- Krueger C. M., Neufeld E. J., Saffitz J. E. Preservation of arachidonoyl phospholipids during tissue processing for electron microscopic autoradiography. J Histochem Cytochem. 1985 Aug;33(8):799–802. doi: 10.1177/33.8.3926867. [DOI] [PubMed] [Google Scholar]
- Longworth D. L., Foster D. W., Dvorak A. M., Weller P. F. Incorporation of arachidonic acid by microfilariae of Brugia malayi. J Infect Dis. 1985 Dec;152(6):1317–1323. doi: 10.1093/infdis/152.6.1317. [DOI] [PubMed] [Google Scholar]
- Lutas E. M., Zucker-Franklin D. Formation of lipid inclusions in normal human leukocytes. Blood. 1977 Feb;49(2):309–320. [PubMed] [Google Scholar]
- Nissen H. P., Kreysel H. W. Analysis of phospholipids in human semen by high-performance liquid chromatography. J Chromatogr. 1983 Aug 12;276(1):29–35. doi: 10.1016/s0378-4347(00)85062-8. [DOI] [PubMed] [Google Scholar]
- Robinson J. M., Karnovsky M. L., Karnovsky M. J. Glycogen accumulation in polymorphonuclear leukocytes, and other intracellular alterations that occur during inflammation. J Cell Biol. 1982 Dec;95(3):933–942. doi: 10.1083/jcb.95.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen C. L., Chilton F. H., O'Flaherty J. T., Surles J. R., Piantadosi C., Waite M., Wykle R. L. Human neutrophils incorporate arachidonic acid and saturated fatty acids into separate molecular species of phospholipids. Biochim Biophys Acta. 1987 May 13;919(1):79–89. doi: 10.1016/0005-2760(87)90220-7. [DOI] [PubMed] [Google Scholar]
- Weller P. F., Dvorak A. M. Arachidonic acid incorporation by cytoplasmic lipid bodies of human eosinophils. Blood. 1985 May;65(5):1269–1274. [PubMed] [Google Scholar]