Abstract
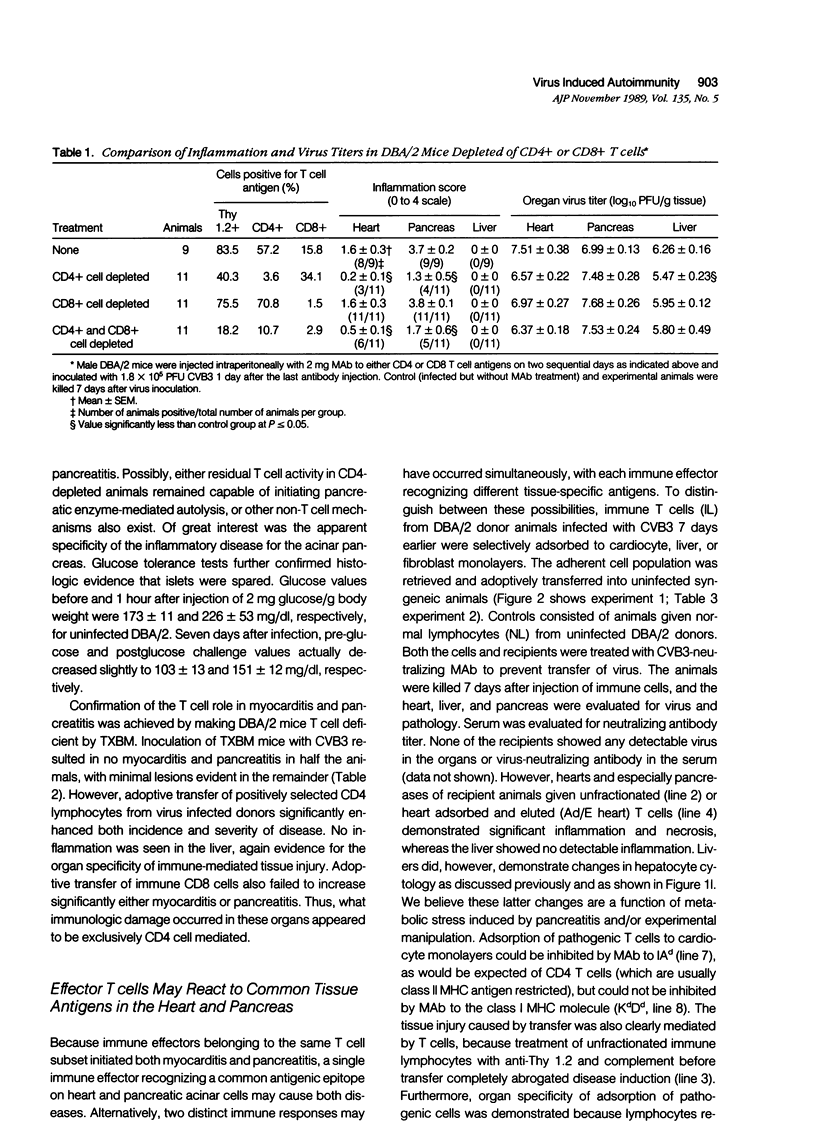
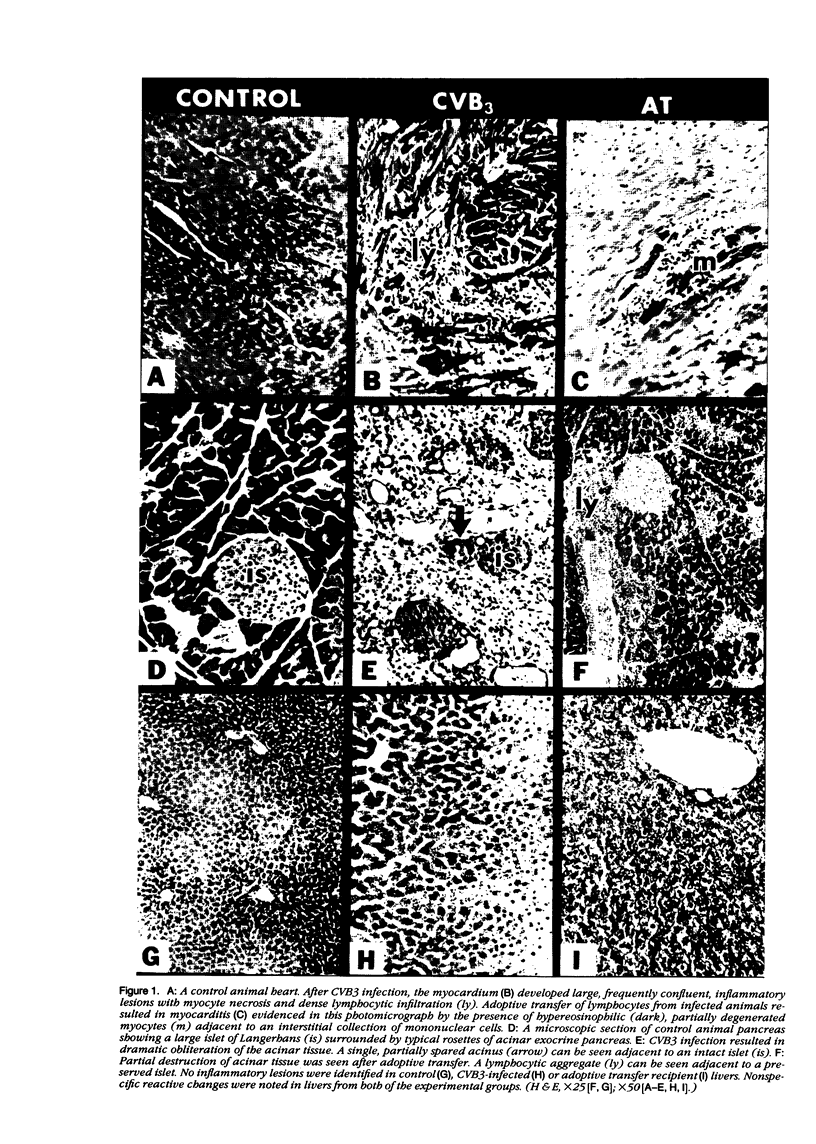
DBA/2 male mice inoculated intraperitoneally with 1.8 X 10(5) plaque-forming units (PFU) coxsackievirus B-3 (CVB3) showed extensive inflammatory cell infiltration of the myocardium and acinar tissue of the pancreas in 7 days. Selective depletion of T lymphocyte subpopulations indicated that CD4 cells were either completely or partially responsible for cell damage in both organs. Other organs such as the liver were infected and contained virus titers equivalent to those seen in the heart and pancreas but showed no apparent tissue injury. The role of the CD4 cell was confirmed by positive selection of either T cell subpopulation from CVB3-immune lymphocytes in vitro and adoptive transfer of these cells into T cell-deficient (thymectomized, irradiated, bone marrow reconstituted, TXBM) DBA/2 recipients. Lymphocytes from CVB3-infected donor mice were adsorbed to myocyte, skin fibroblast, or liver vascular endothelial cell (VEC) monolayers. The adherent population was retrieved and adoptively transferred into uninfected syngeneic recipients. When killed 7 days later, the animals receiving unfractionated immune lymphocytes or cells eluted from heart monolayers developed both myocarditis and pancreatitis. Anti-Thy 1.2 and C' treatment of the unfractionated cells completely abrogated transfer of disease. Cells eluted from either fibroblast or liver VEC monolayers showed no pathogenicity. Adsorption of immune cells to heart monolayers in the presence of anti-IAd (class II major histocompatibility complex antigen, MHC) inhibited attachment of the pathogenic T cell, whereas anti KdDd (a class I MHC antigen) had no effect.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Salmi A., Butler L. D., Chiller J. M., Oldstone M. B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984 Aug 1;160(2):521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu P. G., Huber S., Sriram S., Craighead J. E. Genetic control of multisystem autoimmune disease in encephalomyocarditis virus infected BALB/cCUM and BALB/cBYJ mice. Curr Top Microbiol Immunol. 1985;122:154–161. doi: 10.1007/978-3-642-70740-7_23. [DOI] [PubMed] [Google Scholar]
- Ben-Nun A., Cohen I. R. Experimental autoimmune encephalomyelitis (EAE) mediated by T cell lines: process of selection of lines and characterization of the cells. J Immunol. 1982 Jul;129(1):303–308. [PubMed] [Google Scholar]
- Bottazzo G. F., Dean B. M., McNally J. M., MacKay E. H., Swift P. G., Gamble D. R. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985 Aug 8;313(6):353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- Bowles N. E., Dubowitz V., Sewry C. A., Archard L. C. Dermatomyositis, polymyositis, and Coxsackie-B-virus infection. Lancet. 1987 May 2;1(8540):1004–1007. doi: 10.1016/s0140-6736(87)92271-9. [DOI] [PubMed] [Google Scholar]
- Crowell R. L., Field A. K., Schleif W. A., Long W. L., Colonno R. J., Mapoles J. E., Emini E. A. Monoclonal antibody that inhibits infection of HeLa and rhabdomyosarcoma cells by selected enteroviruses through receptor blockade. J Virol. 1986 Feb;57(2):438–445. doi: 10.1128/jvi.57.2.438-445.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. W., Hall N. K., Krisher K. K., Spanier A. M. A study of anti-group A streptococcal monoclonal antibodies cross-reactive with myosin. J Immunol. 1986 Jan;136(1):293–298. [PubMed] [Google Scholar]
- Eaton M. D., Almquist S. J. Autoimmunity induced by injection of virus-modified cell membrane antigens in syngeneic mice. Infect Immun. 1977 Jan;15(1):322–328. doi: 10.1128/iai.15.1.322-328.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton M. D. Autoimmunity induced by syngeneic splenocyte membranes carrying irreversibly adsorbed paramyxovirus. Infect Immun. 1980 Mar;27(3):855–861. doi: 10.1128/iai.27.3.855-861.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlanger B. F., Cleveland W. L., Wassermann N. H., Ku H. H., Hill B. L., Sarangarajan R., Rajagopalan R., Cayanis E., Edelman I. S., Penn A. S. Auto-anti-idiotype: a basis for autoimmunity and a strategy for anti-receptor antibodies. Immunol Rev. 1986 Dec;94:23–37. doi: 10.1111/j.1600-065x.1986.tb01162.x. [DOI] [PubMed] [Google Scholar]
- Germain R. N. Immunology. The ins and outs of antigen processing and presentation. Nature. 1986 Aug 21;322(6081):687–689. doi: 10.1038/322687a0. [DOI] [PubMed] [Google Scholar]
- Griffiths M. M., DeWitt C. W. Genetic control of collagen-induced arthritis in rats: the immune response to type II collagen among susceptible and resistant strains and evidence for multiple gene control. J Immunol. 1984 Jun;132(6):2830–2836. [PubMed] [Google Scholar]
- Hsu K. H., Lonberg-Holm K., Alstein B., Crowell R. L. A monoclonal antibody specific for the cellular receptor for the group B coxsackieviruses. J Virol. 1988 May;62(5):1647–1652. doi: 10.1128/jvi.62.5.1647-1652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. K., Sriram S. Clonal diversity of myelin basic protein-specific T lymphocytes. Immunogenetics. 1988;27(5):370–374. doi: 10.1007/BF00395133. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Job L. P. Cellular immune mechanisms in Coxsackievirus group B, type 3 induced myocarditis in Balb/C mice. Adv Exp Med Biol. 1983;161:491–508. doi: 10.1007/978-1-4684-4472-8_29. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Lodge P. A. Coxsackievirus B-3 myocarditis in Balb/c mice. Evidence for autoimmunity to myocyte antigens. Am J Pathol. 1984 Jul;116(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- Lyden D. C., Olszewski J., Feran M., Job L. P., Huber S. A. Coxsackievirus B-3-induced myocarditis. Effect of sex steroids on viremia and infectivity of cardiocytes. Am J Pathol. 1987 Mar;126(3):432–438. [PMC free article] [PubMed] [Google Scholar]
- Marriott S. J., Roeder D. J., Consigli R. A. Anti-idiotypic antibodies to a polyomavirus monoclonal antibody recognize cell surface components of mouse kidney cells and prevent polyomavirus infection. J Virol. 1987 Sep;61(9):2747–2753. doi: 10.1128/jvi.61.9.2747-2753.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera T., Toniolo A., Ray U. R., Jenson A. B., Knazek R. A., Notkins A. L. Virus-induced diabetes mellitus. XX. Polyendocrinopathy and autoimmunity. J Exp Med. 1981 Jun 1;153(6):1457–1473. doi: 10.1084/jem.153.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotz P. H. Autoantibodies are anti-idiotype antibodies to antiviral antibodies. Lancet. 1983 Oct 8;2(8354):824–826. doi: 10.1016/s0140-6736(83)90740-7. [DOI] [PubMed] [Google Scholar]
- Ray C. G., Minnich L. L., Johnson P. C. Selective polymyositis inducted by coxsackievirus B1 in mice. J Infect Dis. 1979 Aug;140(2):239–243. doi: 10.1093/infdis/140.2.239. [DOI] [PubMed] [Google Scholar]
- Saegusa J., Prabhakar B. S., Essani K., McClintock P. R., Fukuda Y., Ferrans V. J., Notkins A. L. Monoclonal antibody to coxsackievirus B4 reacts with myocardium. J Infect Dis. 1986 Feb;153(2):372–373. doi: 10.1093/infdis/153.2.372. [DOI] [PubMed] [Google Scholar]
- Sun N. C., Smith V. M. Hepatitis associated with myocarditis. Unusual manifestation of infection with Coxsackie virus group B, type 3. N Engl J Med. 1966 Jan 27;274(4):190–193. doi: 10.1056/NEJM196601272740405. [DOI] [PubMed] [Google Scholar]
- Weigle W. O. Analysis of autoimmunity through experimental models of thyroiditis and allergic encephalomyelitis. Adv Immunol. 1980;30:159–273. doi: 10.1016/s0065-2776(08)60196-0. [DOI] [PubMed] [Google Scholar]
- Wolfgram L. J., Beisel K. W., Rose N. R. Heart-specific autoantibodies following murine coxsackievirus B3 myocarditis. J Exp Med. 1985 May 1;161(5):1112–1121. doi: 10.1084/jem.161.5.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Involvement of T lymphocytes in the pathogenesis of coxsackie virus B3 heart disease. J Immunol. 1974 Dec;113(6):1726–1734. [PubMed] [Google Scholar]
- Ytterberg S. R., Mahowald M. L., Messner R. P. Coxsackievirus B 1-induced polymyositis. Lack of disease expression in nu/nu mice. J Clin Invest. 1987 Aug;80(2):499–506. doi: 10.1172/JCI113098. [DOI] [PMC free article] [PubMed] [Google Scholar]




