Abstract
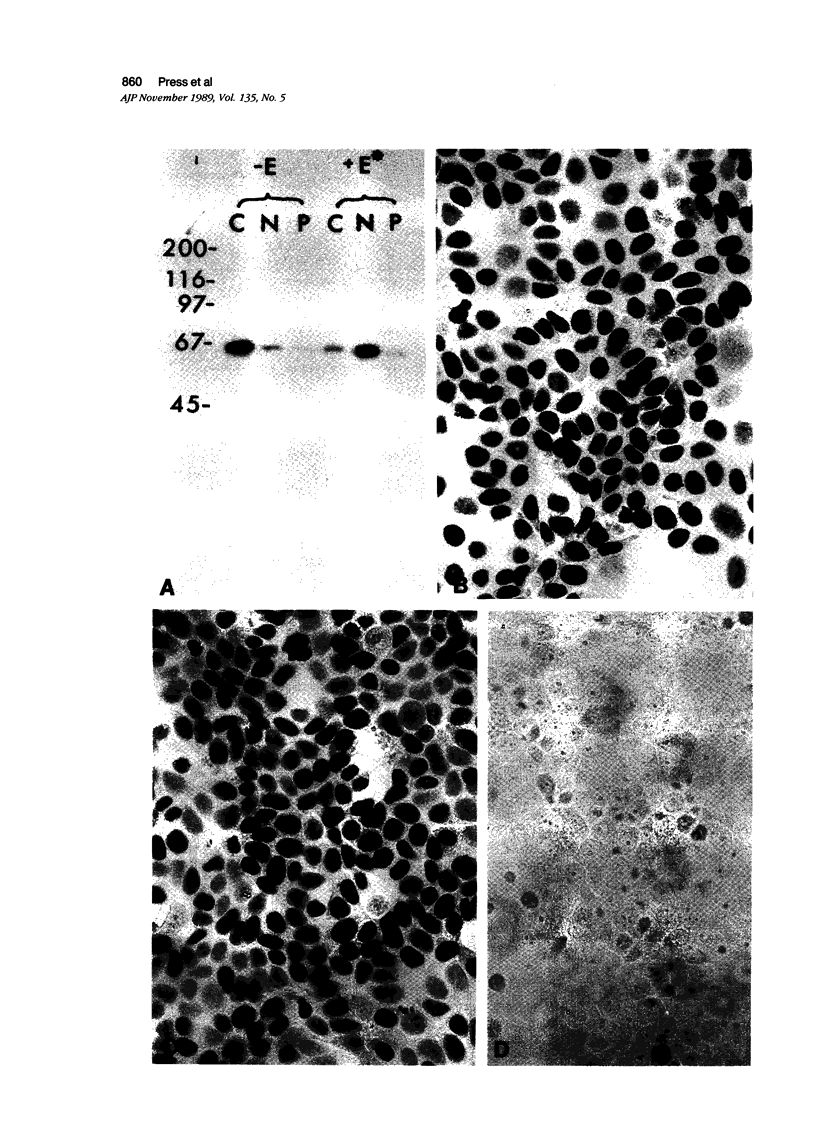
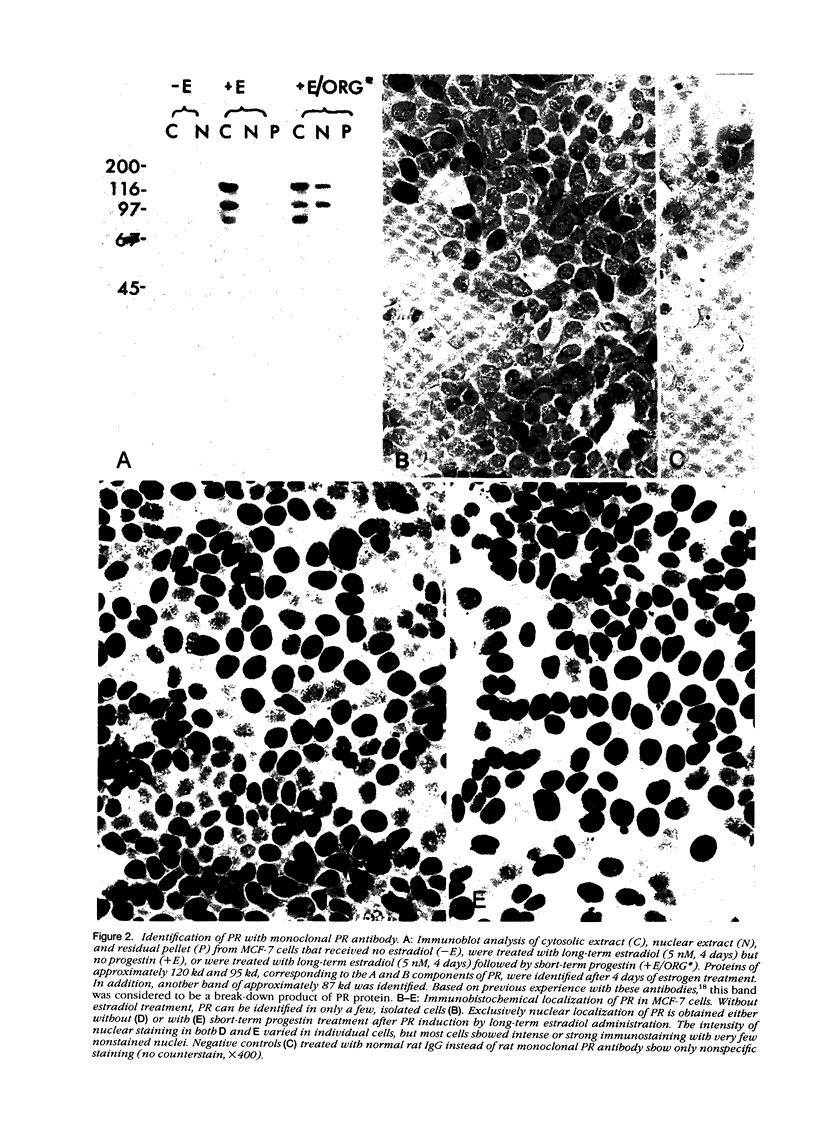
The estrogen receptor (ER) and progesterone receptor (PR) content of cultured human breast carcinoma cells (MCF-7) was determined by biochemical assay, immunoblot analysis, and immunohistochemical assay under varying conditions of hormonal stimulation. The ER and PR content in cytosolic and nuclear extracts varied with steroid treatment. However, both the amount and distribution of each receptor in these extracts was virtually the same when determined by steroid binding and immunoblot analyses. Two immunocytochemical parameters (staining intensity and proportion of cells stained) correlated with the quantitative analyses of ER and PR, but not with the subcellular distribution. When MCF-7 cells were grown for 4 days in charcoal-stripped serum without phenol red, 93% of total ER was found in the cytosol (10 mM KCl), whereas short-term treatment with 5 nM estradiol resulted in the appearance of 82% of total ER in the nuclear extract (400 mM KCl). With either cell treatment only nuclear staining for ER was observed. Progesterone receptor was virtually undetectable in the same cells by any method. After 4 days of treatment by 5 nM estradiol, PR was strongly induced (50-fold) in MCF-7 cells as determined by all three methods. As observed for ER, 95% of total induced PR was found in the cytosol in the absence of a progestin. Short-term treatment with 5 nM ORG 2058, a synthetic progestin, resulted in the appearance of 42% of total PR in the nuclear extract. However, only strong nuclear staining for PR was observed in either the presence or absence of a progestin. These findings are consistent with the current view of ER and PR as nuclear receptors present in at least two forms. One of these, the unoccupied form of the receptor, is easily removed from the nucleus by hypotonic buffers during the cell homogenization process and appears in the cytosolic extract. The other form of the receptor, the steroid-occupied form, is more tightly bound to nuclear components and is removed from nuclei only under more vigorous extraction conditions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berthois Y., Katzenellenbogen J. A., Katzenellenbogen B. S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Goeij A. F., Volleberg M. P., Hondius G. G., Bosman F. T. Radiochemical determination of estrogen receptors in cryostat sections of target tissues. J Steroid Biochem. 1984 Aug;21(2):127–134. doi: 10.1016/0022-4731(84)90372-8. [DOI] [PubMed] [Google Scholar]
- Eckert R. L., Katzenellenbogen B. S. Effects of estrogens and antiestrogens on estrogen receptor dynamics and the induction of progesterone receptor in MCF-7 human breast cancer cells. Cancer Res. 1982 Jan;42(1):139–144. [PubMed] [Google Scholar]
- Gormley G. J., Hospelhorn V. D., Khan M. K., Jensen E. V. A controlled pore glass bead assay for the measurement of cytoplasmic and nuclear glucocorticoid receptors. J Steroid Biochem. 1985 Jun;22(6):693–698. doi: 10.1016/0022-4731(85)90273-0. [DOI] [PubMed] [Google Scholar]
- Gorski J., Toft D., Shyamala G., Smith D., Notides A. Hormone receptors: studies on the interaction of estrogen with the uterus. Recent Prog Horm Res. 1968;24:45–80. doi: 10.1016/b978-1-4831-9827-9.50008-3. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. Oestradiol induction of a glucocorticoid-responsive gene by a chimaeric receptor. Nature. 1987 Jan 1;325(6099):75–78. doi: 10.1038/325075a0. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Harris K., Bova R., Kinders R., Moore B., Nolan C. Purification of T47D human progesterone receptor and immunochemical characterization with monoclonal antibodies. Mol Endocrinol. 1988 Aug;2(8):714–726. doi: 10.1210/mend-2-8-714. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Nolan C., Engler J. P., Jensen E. V. Monoclonal antibodies to human estrogen receptor. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5115–5119. doi: 10.1073/pnas.77.9.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene G. L., Sobel N. B., King W. J., Jensen E. V. Immunochemical studies of estrogen receptors. J Steroid Biochem. 1984 Jan;20(1):51–56. doi: 10.1016/0022-4731(84)90188-2. [DOI] [PubMed] [Google Scholar]
- Grody W. W., Schrader W. T., O'Malley B. W. Activation, transformation, and subunit structure of steroid hormone receptors. Endocr Rev. 1982 Spring;3(2):141–163. doi: 10.1210/edrv-3-2-141. [DOI] [PubMed] [Google Scholar]
- Holt J. A., Lorincz M. A., Hospelhorn V. D. Sulfhydryl sensitivity and [125I]-16 alpha-IODO-17 beta-estradiol binding of estrogen receptor in ovarian epithelial carcinomas. J Steroid Biochem. 1983 Jan;18(1):41–50. doi: 10.1016/0022-4731(83)90328-x. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., McGuire W. L. Estrogen control of progesterone receptor in human breast cancer. Correlation with nuclear processing of estrogen receptor. J Biol Chem. 1978 Apr 10;253(7):2223–2228. [PubMed] [Google Scholar]
- Jensen E. V., Suzuki T., Kawashima T., Stumpf W. E., Jungblut P. W., DeSombre E. R. A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):632–638. doi: 10.1073/pnas.59.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis J. A., Walent J. H., Gorski J. Estrogen receptors in rat uterine cell cultures: effects of medium on receptor concentration. Endocrinology. 1984 Aug;115(2):762–769. doi: 10.1210/endo-115-2-762. [DOI] [PubMed] [Google Scholar]
- King W. J., Greene G. L. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984 Feb 23;307(5953):745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Lubahn D. B., Joseph D. R., Sar M., Tan J., Higgs H. N., Larson R. E., French F. S., Wilson E. M. The human androgen receptor: complementary deoxyribonucleic acid cloning, sequence analysis and gene expression in prostate. Mol Endocrinol. 1988 Dec;2(12):1265–1275. doi: 10.1210/mend-2-12-1265. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Schwartz R. J., Schrader W. T. A review of regulation of gene expression by steroid hormone receptors. J Steroid Biochem. 1976 Nov-Dec;7(11-12):1151–1159. doi: 10.1016/0022-4731(76)90048-0. [DOI] [PubMed] [Google Scholar]
- Perrot-Applanat M., Logeat F., Groyer-Picard M. T., Milgrom E. Immunocytochemical study of mammalian progesterone receptor using monoclonal antibodies. Endocrinology. 1985 Apr;116(4):1473–1484. doi: 10.1210/endo-116-4-1473. [DOI] [PubMed] [Google Scholar]
- Press M. F., Greene G. L. An immunocytochemical method for demonstrating estrogen receptor in human uterus using monoclonal antibodies to human estrophilin. Lab Invest. 1984 Apr;50(4):480–486. [PubMed] [Google Scholar]
- Press M. F., Greene G. L. Localization of progesterone receptor with monoclonal antibodies to the human progestin receptor. Endocrinology. 1988 Mar;122(3):1165–1175. doi: 10.1210/endo-122-3-1165. [DOI] [PubMed] [Google Scholar]
- Press M. F., Holt J. A., Herbst A. L., Greene G. L. Immunocytochemical identification of estrogen receptor in ovarian carcinomas. Localization with monoclonal estrophilin antibodies compared with biochemical assays. Lab Invest. 1985 Sep;53(3):349–361. [PubMed] [Google Scholar]
- Press M. F., Nousek-Goebl N., King W. J., Herbst A. L., Greene G. L. Immunohistochemical assessment of estrogen receptor distribution in the human endometrium throughout the menstrual cycle. Lab Invest. 1984 Nov;51(5):495–503. [PubMed] [Google Scholar]
- Press M. F., Udove J. A., Greene G. L. Progesterone receptor distribution in the human endometrium. Analysis using monoclonal antibodies to the human progesterone receptor. Am J Pathol. 1988 Apr;131(1):112–124. [PMC free article] [PubMed] [Google Scholar]
- Read L. D., Snider C. E., Miller J. S., Greene G. L., Katzenellenbogen B. S. Ligand-modulated regulation of progesterone receptor messenger ribonucleic acid and protein in human breast cancer cell lines. Mol Endocrinol. 1988 Mar;2(3):263–271. doi: 10.1210/mend-2-3-263. [DOI] [PubMed] [Google Scholar]
- Ree A. H., Landmark B. F., Eskild W., Levy F. O., Lahooti H., Jahnsen T., Aakvaag A., Hansson V. Autologous down-regulation of messenger ribonucleic acid and protein levels for estrogen receptors in MCF-7 cells: an inverse correlation to progesterone receptor levels. Endocrinology. 1989 May;124(5):2577–2583. doi: 10.1210/endo-124-5-2577. [DOI] [PubMed] [Google Scholar]
- Stefanini M., De Martino C., Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature. 1967 Oct 14;216(5111):173–174. doi: 10.1038/216173a0. [DOI] [PubMed] [Google Scholar]
- Tan J. A., Joseph D. R., Quarmby V. E., Lubahn D. B., Sar M., French F. S., Wilson E. M. The rat androgen receptor: primary structure, autoregulation of its messenger ribonucleic acid, and immunocytochemical localization of the receptor protein. Mol Endocrinol. 1988 Dec;2(12):1276–1285. doi: 10.1210/mend-2-12-1276. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L. L., Krett N. L., Francis M. D., Gordon D. F., Wood W. M., O'Malley B. W., Horwitz K. B. Multiple human progesterone receptor messenger ribonucleic acids and their autoregulation by progestin agonists and antagonists in breast cancer cells. Mol Endocrinol. 1988 Jan;2(1):62–72. doi: 10.1210/mend-2-1-62. [DOI] [PubMed] [Google Scholar]
- Welshons W. V., Krummel B. M., Gorski J. Nuclear localization of unoccupied receptors for glucocorticoids, estrogens, and progesterone in GH3 cells. Endocrinology. 1985 Nov;117(5):2140–2147. doi: 10.1210/endo-117-5-2140. [DOI] [PubMed] [Google Scholar]
- Welshons W. V., Lieberman M. E., Gorski J. Nuclear localization of unoccupied oestrogen receptors. Nature. 1984 Feb 23;307(5953):747–749. doi: 10.1038/307747a0. [DOI] [PubMed] [Google Scholar]