Abstract
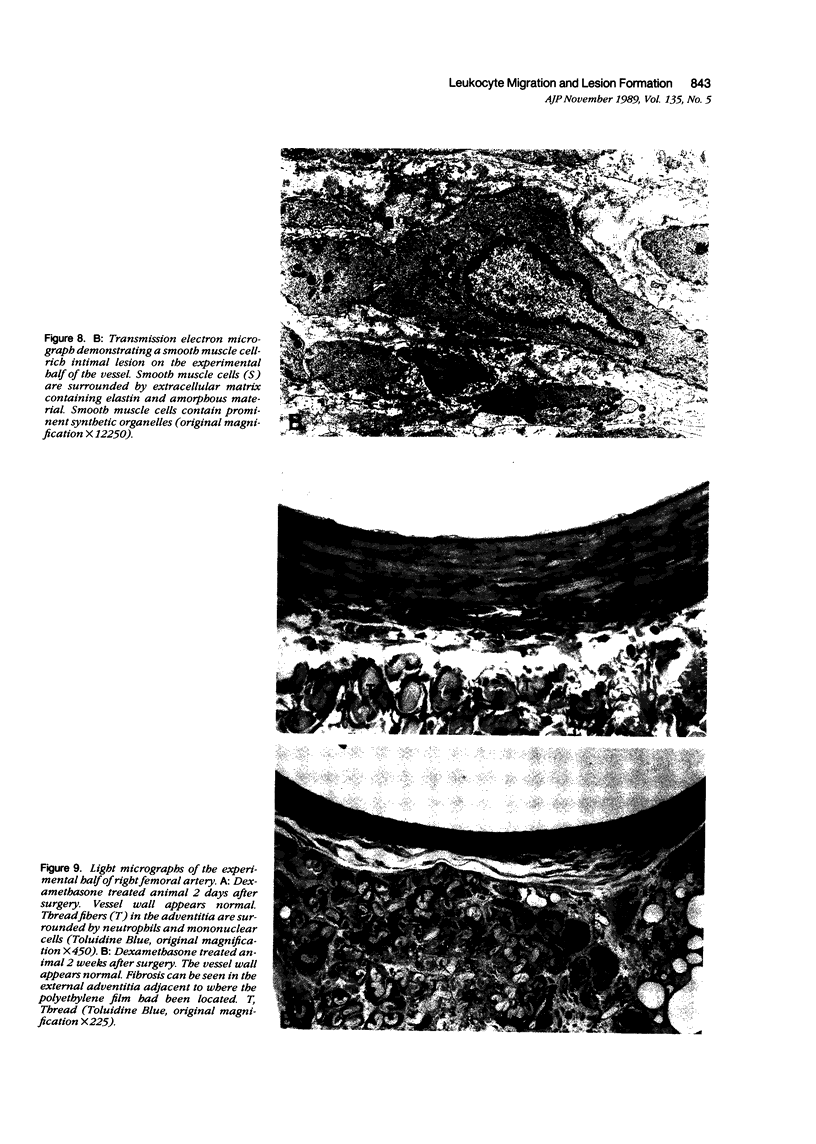
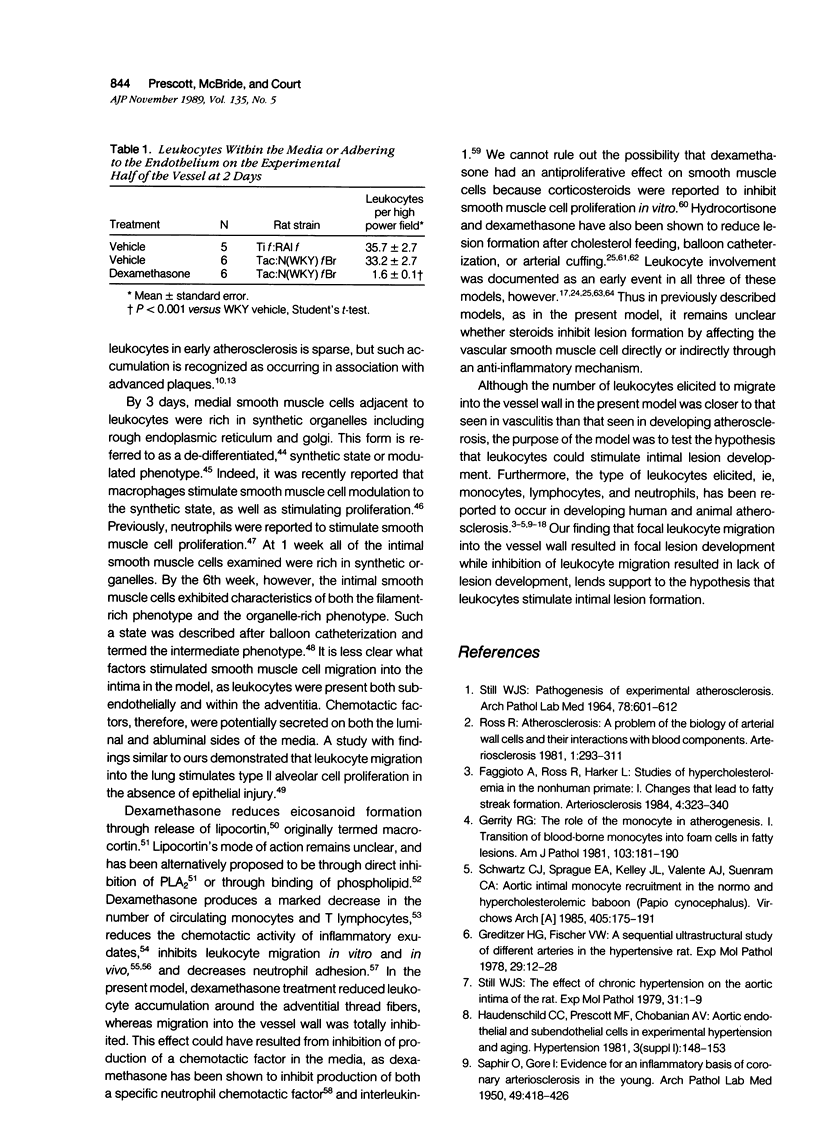
A model was developed to study the role of leukocytes in the development of vascular lesions. Implantation of an endotoxin-soaked cotton thread in the adventitia on the ventral side of the rat femoral artery resulted in leukocyte migration into the vessel wall exclusively in the ventral half of the vessel. Leukocyte migration occurred from both the luminal and adventitial side and consisted of neutrophils and mononuclear cells. Smooth muscle cell rich intimal lesions localized to the ventral half of the vessel were first observed 1 week after implantation. Lesions remained localized to the ventral half of the vessel wall through the 6th week. When leukocyte migration into the vessel wall was inhibited by treatment with dexamethasone, lesion development did not occur. These results suggest that leukocytes can stimulate smooth muscle cell migration into the intima and result in intimal lesion formation.
Full text
PDF


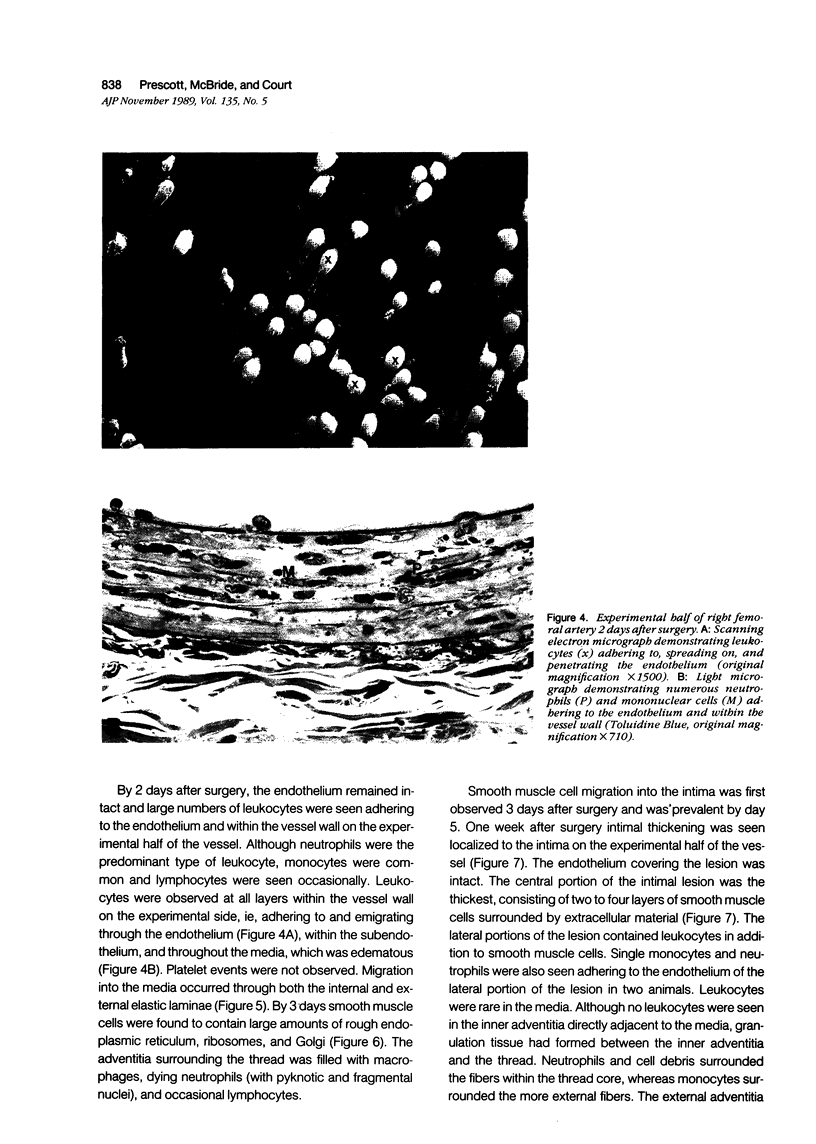

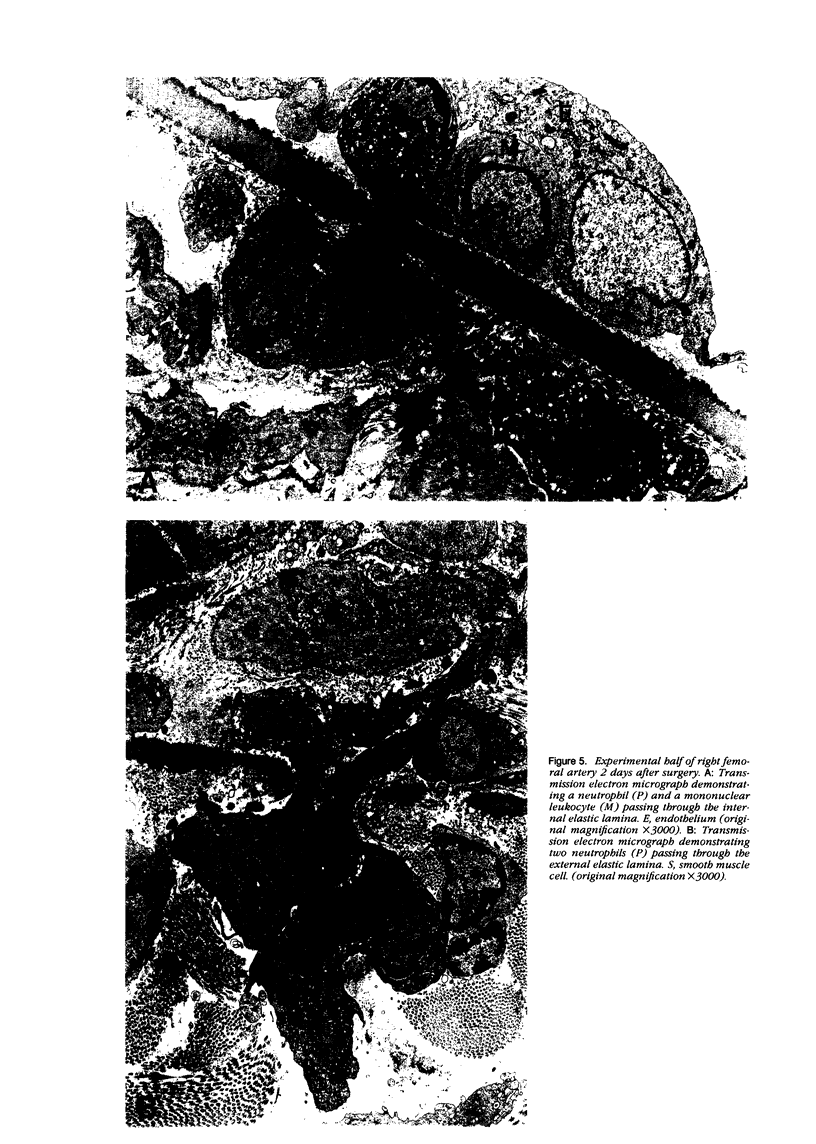

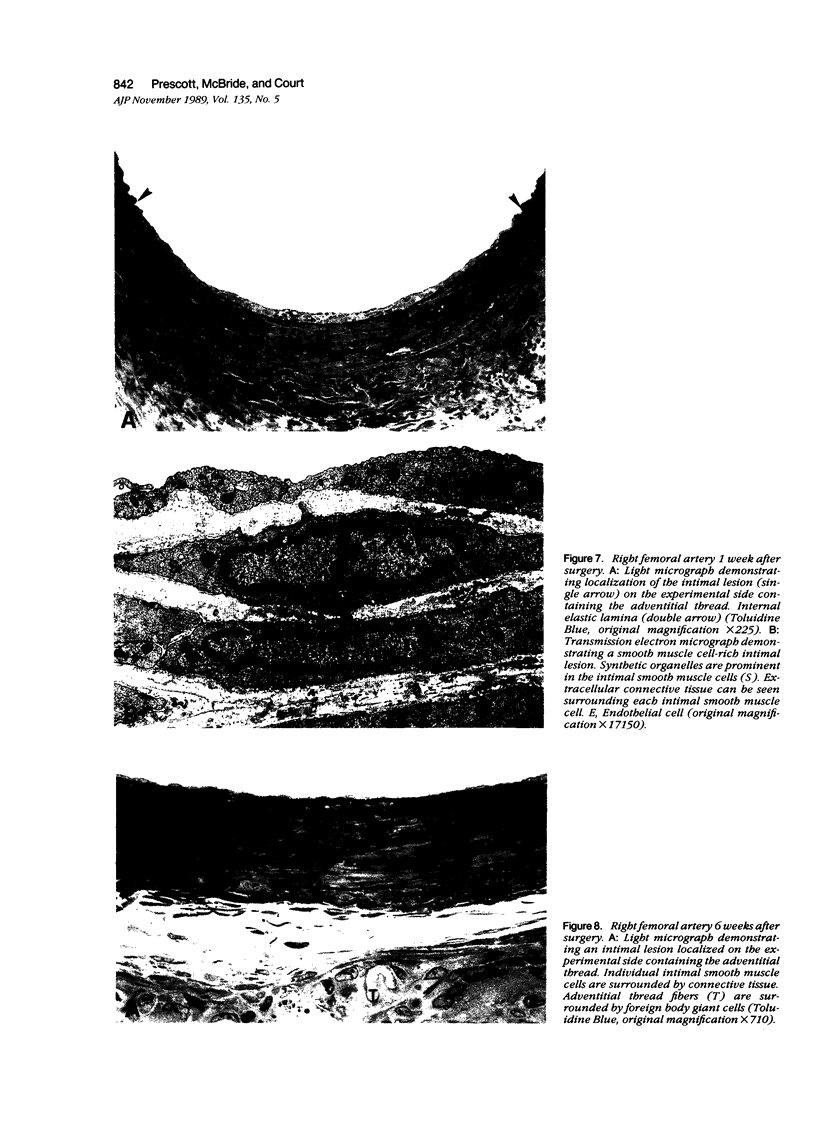


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach R., Sidky Y. A. Nature of the stimulus leading to lymphocyte-induced angiogenesis. J Immunol. 1979 Aug;123(2):751–754. [PubMed] [Google Scholar]
- Bailey J. M., Butler J. Anti-inflammatory drugs in experimental atherosclerosis. I. Relative potencies for inhibiting plaque formation. Atherosclerosis. 1973 May-Jun;17(3):515–522. doi: 10.1016/0021-9150(73)90041-5. [DOI] [PubMed] [Google Scholar]
- Björkerud S. Reaction of the aortic wall of the rabbit after superficial, longitudinal, mechanical trauma. Virchows Arch A Pathol Pathol Anat. 1969;347(3):197–210. doi: 10.1007/BF00543107. [DOI] [PubMed] [Google Scholar]
- Blackwell G. J., Carnuccio R., Di Rosa M., Flower R. J., Langham C. S., Parente L., Persico P., Russel-Smith N. C., Stone D. Glucocorticoids induce the formation and release of anti-inflammatory and anti-phospholipase proteins into the peritoneal cavity of the rat. Br J Pharmacol. 1982 May;76(1):185–194. doi: 10.1111/j.1476-5381.1982.tb09205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKSON T. B., PRICHARD R. W., NETSKY M. G., LOFLAND H. B. Atherosclerosis in pigeons; its spontaneous occurrence and resemblance to human atherosclerosis. AMA Arch Pathol. 1959 Aug;68(2):143–147. [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Cliff W. J., Heathcote C. R., Moss N. S., Reichenbach D. D. The coronary arteries in cases of cardiac and noncardiac sudden death. Am J Pathol. 1988 Aug;132(2):319–329. [PMC free article] [PubMed] [Google Scholar]
- Colditz I. G., Movat H. Z. Kinetics of neutrophil accumulation in acute inflammatory lesions induced by chemotaxins and chemotaxinigens. J Immunol. 1984 Oct;133(4):2169–2173. [PubMed] [Google Scholar]
- Cole C. W., Hagen P. O., Lucas J. F., Mikat E. M., O'Malley M. K., Radic Z. S., Makhoul R. G., McCann R. L. Association of polymorphonuclear leukocytes with sites of aortic catheter-induced injury in rabbits. Atherosclerosis. 1987 Oct;67(2-3):229–236. doi: 10.1016/0021-9150(87)90283-8. [DOI] [PubMed] [Google Scholar]
- Cunha F. Q., Ferreira S. H. The release of a neutrophil chemotactic factor from peritoneal macrophages by endotoxin: inhibition by glucocorticoids. Eur J Pharmacol. 1986 Sep 23;129(1-2):65–76. doi: 10.1016/0014-2999(86)90337-7. [DOI] [PubMed] [Google Scholar]
- Cupps T. R., Fauci A. S. Corticosteroid-mediated immunoregulation in man. Immunol Rev. 1982;65:133–155. doi: 10.1111/j.1600-065x.1982.tb00431.x. [DOI] [PubMed] [Google Scholar]
- DUGUID J. B. Diet and coronary disease. Lancet. 1954 May 1;266(6818):891–895. doi: 10.1016/s0140-6736(54)91521-4. [DOI] [PubMed] [Google Scholar]
- Davidson F. F., Dennis E. A., Powell M., Glenney J. R., Jr Inhibition of phospholipase A2 by "lipocortins" and calpactins. An effect of binding to substrate phospholipids. J Biol Chem. 1987 Feb 5;262(4):1698–1705. [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Flower R. J. Background and discovery of lipocortins. Agents Actions. 1986 Jan;17(3-4):255–262. doi: 10.1007/BF01982616. [DOI] [PubMed] [Google Scholar]
- Fritz K. E., Jarmolych J., Daoud A. S. Association of DNA synthesis and apparent dedifferentiation of aortic smooth muscle cells in vitro. Exp Mol Pathol. 1970 Jun;12(3):354–362. doi: 10.1016/0014-4800(70)90066-3. [DOI] [PubMed] [Google Scholar]
- Fromer C. H., Klintworth G. K. An evaluation of the role of leukocytes in the pathogenesis of experimentally induced corneal vascularization. III. Studies related to the vasoproliferative capability of polymorphonuclear leukocytes and lymphocytes. Am J Pathol. 1976 Jan;82(1):157–170. [PMC free article] [PubMed] [Google Scholar]
- Gebrane J., Roland J., Orcel L. Experimental diffuse intimal thickening of the femoral arteries in the rabbit. Virchows Arch A Pathol Anat Histol. 1982;396(1):41–59. doi: 10.1007/BF00428499. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Greditzer H. G., 3rd, Fischer V. W. A sequential ultrastructural study of different arteries in the hypertensive rat. Exp Mol Pathol. 1978 Aug;29(1):12–28. doi: 10.1016/0014-4800(78)90022-9. [DOI] [PubMed] [Google Scholar]
- Grünwald J., Fingerle J., Hämmerle H., Betz E., Haudenschild C. C. Cytocontractile structures and proteins of smooth muscle cells during the formation of experimental lesions. Exp Mol Pathol. 1987 Feb;46(1):78–88. doi: 10.1016/0014-4800(87)90032-3. [DOI] [PubMed] [Google Scholar]
- Helin P., Lorenzen I., Garbarsch C., Matthiessen M. E. Arteriosclerosis in rabbit aorta induced by mechanical dilatation. Biochemical and morphological studies. Atherosclerosis. 1971 May-Jun;13(3):319–331. doi: 10.1016/0021-9150(71)90075-x. [DOI] [PubMed] [Google Scholar]
- Hirosumi J., Nomoto A., Ohkubo Y., Sekiguchi C., Mutoh S., Yamaguchi I., Aoki H. Inflammatory responses in cuff-induced atherosclerosis in rabbits. Atherosclerosis. 1987 Apr;64(2-3):243–254. doi: 10.1016/0021-9150(87)90252-8. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Glazier A. J., Monick M. M., Dinarello C. A. Interleukin-1 is a chemotactic factor for human T-lymphocytes. Am Rev Respir Dis. 1987 Jan;135(1):66–71. doi: 10.1164/arrd.1987.135.1.66. [DOI] [PubMed] [Google Scholar]
- Hurley J. V., Ryan G. B., Friedman A. The mononuclear response to intrapleural injection in the rat. J Pathol Bacteriol. 1966 Apr;91(2):575–587. doi: 10.1002/path.1700910234. [DOI] [PubMed] [Google Scholar]
- Huth F., Kojimahara M., Franken T., Rhedin P., Rosenbauer K. A. Aortic alterations in rabbits following sheathing with silastic and polyethylene tubes. Curr Top Pathol. 1975;60:1–32. doi: 10.1007/978-3-642-66215-7_1. [DOI] [PubMed] [Google Scholar]
- Issekutz A. C. Vascular responses during acute neutrophilic inflammation. Their relationship to in vivo neutrophil emigration. Lab Invest. 1981 Nov;45(5):435–441. [PubMed] [Google Scholar]
- Jonasson L., Holm J., Hansson G. K. Cyclosporin A inhibits smooth muscle proliferation in the vascular response to injury. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2303–2306. doi: 10.1073/pnas.85.7.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Hansson G. K. Smooth muscle cells express Ia antigens during arterial response to injury. Lab Invest. 1988 Mar;58(3):310–315. [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Jorgensen L., Packham M. A., Rowsell H. C., Mustard J. F. Deposition of formed elements of blood on the intima and signs of intimal injury in the aorta of rabbit, pig, and man. Lab Invest. 1972 Sep;27(3):341–350. [PubMed] [Google Scholar]
- Jorgensen L., Rowsell H. C., Hovig T., Mustard J. F. Resolution and organization of platelet-rich mural thrombi in carotid arteries of swine. Am J Pathol. 1967 Nov;51(5):681–719. [PMC free article] [PubMed] [Google Scholar]
- Joris I., Zand T., Nunnari J. J., Krolikowski F. J., Majno G. Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. Am J Pathol. 1983 Dec;113(3):341–358. [PMC free article] [PubMed] [Google Scholar]
- Kostis J. B., Turkevich D., Sharp J. Association between leukocyte count and the presence and extent of coronary atherosclerosis as determined by coronary arteriography. Am J Cardiol. 1984 Apr 1;53(8):997–999. doi: 10.1016/0002-9149(84)90624-6. [DOI] [PubMed] [Google Scholar]
- Kurihara A., Ohuchi K., Tsurufuji S. Reduction by dexamethasone of chemotactic activity in inflammatory exudates. Eur J Pharmacol. 1984 May 18;101(1-2):11–16. doi: 10.1016/0014-2999(84)90025-6. [DOI] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Birinyi L. K., Auger K. R., Dinarello C. A. Inducible interleukin-1 gene expression in human vascular smooth muscle cells. J Clin Invest. 1986 Dec;78(6):1432–1438. doi: 10.1172/JCI112732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker J. P., Kilty L. A., Johnson L. K. Glucocorticoid inhibition of vascular smooth muscle cell proliferation: influence of homologous extracellular matrix and serum mitogens. J Cell Biol. 1984 Feb;98(2):534–540. doi: 10.1083/jcb.98.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polverini P. J., Cotran P. S., Gimbrone M. A., Jr, Unanue E. R. Activated macrophages induce vascular proliferation. Nature. 1977 Oct 27;269(5631):804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- Poole J. C., Cromwell S. B., Benditt E. P. Behavior of smooth muscle cells and formation of extracellular structures in the reaction of arterial walls to injury. Am J Pathol. 1971 Mar;62(3):391–414. [PMC free article] [PubMed] [Google Scholar]
- Prescott M. F., Müller K. R. Endothelial regeneration in hypertensive and genetically hypercholesterolemic rats. Arteriosclerosis. 1983 May-Jun;3(3):206–214. doi: 10.1161/01.atv.3.3.206. [DOI] [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial regeneration. III. Time course of intimal changes after small defined injury to rat aortic endothelium. Lab Invest. 1981 Apr;44(4):301–308. [PubMed] [Google Scholar]
- Rennick R. E., Campbell J. H., Campbell G. R. Vascular smooth muscle phenotype and growth behaviour can be influenced by macrophages in vitro. Atherosclerosis. 1988 May;71(1):35–43. doi: 10.1016/0021-9150(88)90300-0. [DOI] [PubMed] [Google Scholar]
- Rivkin I., Foschi G. V., Rosen C. H. Inhibition of in vitro neutrophil chemotaxis and spontaneous motility by anti-inflammatory agents. Proc Soc Exp Biol Med. 1976 Nov;153(2):236–240. doi: 10.3181/00379727-153-39518. [DOI] [PubMed] [Google Scholar]
- Ross R. George Lyman Duff Memorial Lecture. Atherosclerosis: a problem of the biology of arterial wall cells and their interactions with blood components. Arteriosclerosis. 1981 Sep-Oct;1(5):293–311. doi: 10.1161/01.atv.1.5.293. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ C. J., MITCHELL J. R. Cellular infiltration of the human arterial adventitia associated with atheromatous plaques. Circulation. 1962 Jul;26:73–78. doi: 10.1161/01.cir.26.1.73. [DOI] [PubMed] [Google Scholar]
- Schwartz C. J., Sprague E. A., Kelley J. L., Valente A. J., Suenram C. A. Aortic intimal monocyte recruitment in the normo and hypercholesterolemic baboon (Papio cynocephalus). An ultrastructural study: implications in atherogenesis. Virchows Arch A Pathol Anat Histopathol. 1985;405(2):175–191. doi: 10.1007/BF00704370. [DOI] [PubMed] [Google Scholar]
- Shami S. G., Evans M. J., Martinez L. A. Type II cell proliferation related to migration of inflammatory cells into the lung. Exp Mol Pathol. 1986 Jun;44(3):344–352. doi: 10.1016/0014-4800(86)90048-1. [DOI] [PubMed] [Google Scholar]
- Snyder D. S., Unanue E. R. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J Immunol. 1982 Nov;129(5):1803–1805. [PubMed] [Google Scholar]
- Spaet T. H., Tiell M. L., Cintron J., Won J. Selective arterial medial injury fails to produce intimal hyperplasia in experimental animals. Thromb Res. 1982 Jul 15;27(2):205–210. doi: 10.1016/0049-3848(82)90200-6. [DOI] [PubMed] [Google Scholar]
- Stary H. C. Coronary artery fine structure in rhesus monkeys: the early atherosclerotic lesion and its progression. Primates Med. 1976;9:359–395. [PubMed] [Google Scholar]
- Still W. J. The effect of chronic hypertension on the aortic intima of the rat. Exp Mol Pathol. 1979 Aug;31(1):1–9. doi: 10.1016/0014-4800(79)90002-9. [DOI] [PubMed] [Google Scholar]
- Trillo A. A. The cell population of aortic fatty streaks in African green monkeys with special reference to granulocytic cells. An ultrastructural study. Atherosclerosis. 1982 Jun;43(2-3):259–275. doi: 10.1016/0021-9150(82)90027-2. [DOI] [PubMed] [Google Scholar]
- Vane J., Botting R. Inflammation and the mechanism of action of anti-inflammatory drugs. FASEB J. 1987 Aug;1(2):89–96. [PubMed] [Google Scholar]
- Wankowicz Z., Megyeri P., Issekutz A. Synergy between tumour necrosis factor alpha and interleukin-1 in the induction of polymorphonuclear leukocyte migration during inflammation. J Leukoc Biol. 1988 Apr;43(4):349–356. doi: 10.1002/jlb.43.4.349. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Hirata M., Yoshikawa Y., Nagafuchi Y., Toyoshima H., Watanabe T. Role of macrophages in atherosclerosis. Sequential observations of cholesterol-induced rabbit aortic lesion by the immunoperoxidase technique using monoclonal antimacrophage antibody. Lab Invest. 1985 Jul;53(1):80–90. [PubMed] [Google Scholar]
- Webster W. S., Bishop S. P., Geer J. C. Experimental aortic intimal thickening. I. Morphology and source of intimal cells. Am J Pathol. 1974 Aug;76(2):245–264. [PMC free article] [PubMed] [Google Scholar]
- Weight L. M., Noakes T. D. Is running an analog of anorexia?: A survey of the incidence of eating disorders in female distance runners. Med Sci Sports Exerc. 1987 Jun;19(3):213–217. [PubMed] [Google Scholar]
- Zwahlen R. D., Slauson D. O., Neilsen N. R., Clifford C. B. Increased adhesiveness of complement-stimulated neonatal calf neutrophils and its pharmacologic inhibition. J Leukoc Biol. 1987 Jun;41(6):465–473. doi: 10.1002/jlb.41.6.465. [DOI] [PubMed] [Google Scholar]