Abstract
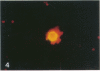
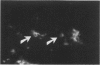
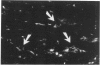
It was recently shown that the human atherosclerotic plaque contains significant amounts of T lymphocytes, and also that smooth muscle cells in these plaques express class II MHC (Ia) antigens. These antigens are not normally present on smooth muscle cells but are inducible by interferon-gamma, a secretory product of activated T cells. Therefore, T cell activation in the plaque was analyzed by immunofluorescent detection of activation markers on T cells isolated from the plaques and in cryostat sections of carotid endarterectomy specimens. Of cells isolated from the plaque, 5% exhibited the E rosettes characteristic of T cells. One third of these cells expressed HLA-DR and VLA-1 (very late activation antigen-1), which in T cells are synthesized only in the activated state. T cells were also identified in sections using immunofluorescent detection of the T cell-specific surface protein, CD3 (Leu-4), with rhodamine labeled second-step antibodies. The frequency of activated T cells was then determined by staining the same, or serial, sections with antibodies to HLA-DR or to the interleukin-2 receptor, followed by biotin-avidin-FITC detection. Of the T cells in the plaque, 34% and 6%, respectively, expressed these cell surface proteins. Taken together, these results indicated that a substantial proportion of the T cells in atherosclerotic plaque are in an activated state. The activation pattern, with a high frequency of HLA-DR and VLA-1 expression and a much lower frequency of interleukin-2 receptor expression, was similar to that reported to occur in chronic inflammatory conditions. Interferon-gamma could be detected in and around some of the lymphocytes, suggesting that paracrine secretion of this lymphokine may occur in the plaque. T cells may be activated locally, presumably by antigen(s) presented in the context of class II MHC expressing smooth muscle cells and/or macrophages, in the atherosclerotic lesion. Such activated T cells may in turn modulate the functions of other cells in the plaque.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellamy A., Davison A. N., Feldmann M. Derivation of ganglioside-specific T cell lines of suppressor or helper phenotype from cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol. 1986 Aug;12(2):107–120. doi: 10.1016/0165-5728(86)90024-x. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Jahn B., Gramatzki M., Zacher J., Kalden J. R. Activated T cells in vivo and in vitro: divergence in expression of Tac and Ia antigens in the nonblastoid small T cells of inflammation and normal T cells activated in vitro. J Immunol. 1984 Sep;133(3):1230–1234. [PubMed] [Google Scholar]
- Chen L. K., Mathieu-Mahul D., Sasportes M., Degos L., Bensussan A. What is a T-cell clone? Effect of rIFN on T-cell clone function and T-cell receptor gene rearrangement. Hum Immunol. 1986 Nov;17(3):214–223. doi: 10.1016/0198-8859(86)90273-9. [DOI] [PubMed] [Google Scholar]
- Emeson E. E., Robertson A. L., Jr T lymphocytes in aortic and coronary intimas. Their potential role in atherogenesis. Am J Pathol. 1988 Feb;130(2):369–376. [PMC free article] [PubMed] [Google Scholar]
- Fox R. I., Fong S., Sabharwal N., Carstens S. A., Kung P. C., Vaughan J. H. Synovial fluid lymphocytes differ from peripheral blood lymphocytes in patients with rheumatoid arthritis. J Immunol. 1982 Jan;128(1):351–354. [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Hancock W. W., Kobzik L., Colby A. J., O'Hara C. J., Cooper A. G., Godleski J. J. Detection of lymphokines and lymphokine receptors in pulmonary sarcoidosis. Immunohistologic evidence that inflammatory macrophages express IL-2 receptors. Am J Pathol. 1986 Apr;123(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Hancock W. W., Muller W. A., Cotran R. S. Interleukin 2 receptors are expressed by alveolar macrophages during pulmonary sarcoidosis and are inducible by lymphokine treatment of normal human lung macrophages, blood monocytes, and monocyte cell lines. J Immunol. 1987 Jan 1;138(1):185–191. [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Holm J., Claesson-Welsh L. Class II MHC antigen expression in the atherosclerotic plaque: smooth muscle cells express HLA-DR, HLA-DQ and the invariant gamma chain. Clin Exp Immunol. 1986 May;64(2):261–268. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Holm J., Clowes M. M., Clowes A. W. Gamma-interferon regulates vascular smooth muscle proliferation and Ia antigen expression in vivo and in vitro. Circ Res. 1988 Oct;63(4):712–719. doi: 10.1161/01.res.63.4.712. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Glass D., Coblyn J. S., Jacobson J. G. Very late activation antigens on rheumatoid synovial fluid T lymphocytes. Association with stages of T cell activation. J Clin Invest. 1986 Sep;78(3):696–702. doi: 10.1172/JCI112629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E., Ware C. F., Strominger J. L. Characterization of a novel differentiation antigen complex recognize by a monoclonal antibody (A-1A5): unique activation-specific molecular forms on stimulated T cells. J Immunol. 1983 Jul;131(1):334–340. [PubMed] [Google Scholar]
- Jonasson L., Holm J., Hansson G. K. Cyclosporin A inhibits smooth muscle proliferation in the vascular response to injury. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2303–2306. doi: 10.1073/pnas.85.7.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Hansson G. K. Smooth muscle cells express Ia antigens during arterial response to injury. Lab Invest. 1988 Mar;58(3):310–315. [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Gabbiani G., Hansson G. K. Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J Clin Invest. 1985 Jul;76(1):125–131. doi: 10.1172/JCI111934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The T cell receptor. Science. 1987 Nov 20;238(4830):1073–1079. doi: 10.1126/science.3317824. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Stegagno M., Wright K. A., Krane S. M., Amento E. P., Colvin R. B., Duquesnoy R. J., Kurnick J. T. Clonal dominance among T-lymphocyte infiltrates in arthritis. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1179–1183. doi: 10.1073/pnas.85.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. Comment on the finding of Ia expression in nonlymphoid cells. Lab Invest. 1986 Aug;55(2):123–125. [PubMed] [Google Scholar]
- Unanue E. R., Beller D. I., Lu C. Y., Allen P. M. Antigen presentation: comments on its regulation and mechanism. J Immunol. 1984 Jan;132(1):1–5. [PubMed] [Google Scholar]
- Waldmann T. A. The structure, function, and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science. 1986 May 9;232(4751):727–732. doi: 10.1126/science.3008337. [DOI] [PubMed] [Google Scholar]