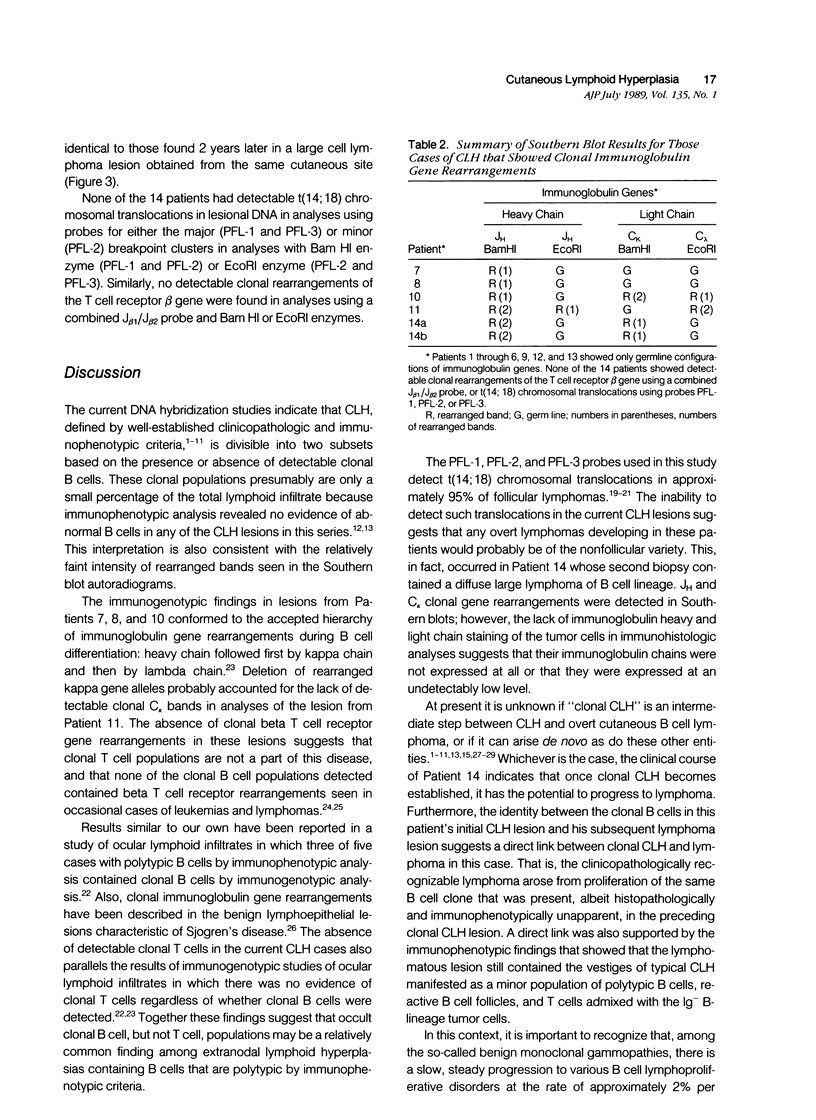
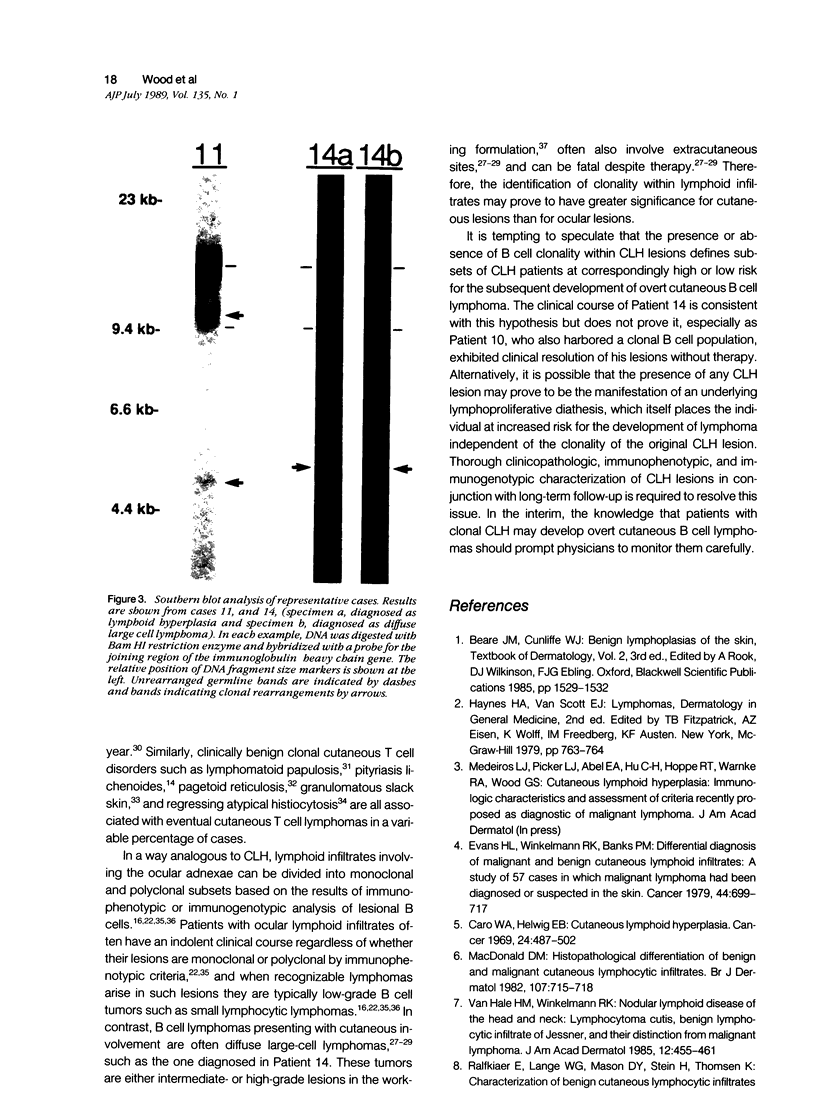
Abstract
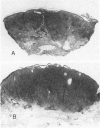
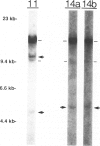
Cutaneous lymphoid hyperplasia (CLH) is a disorder characterized by the development of one or more skin lesions containing dense lymphoid infiltrates that exhibit the histopathologic features of a benign, reactive process. Nevertheless, some cases have been associated with the subsequent development of clinically overt lymphomas. This suggests that monoclonal populations may exist in some cases of CLH and that these cases may represent a subset more likely to evolve into lymphoma. To determine if such a subset of CLH can be distinguished, Southern blot analysis of DNA was used to study the immunogenotypic features of lesions from 14 patients with clinical, histopathologic, and immunopathologic findings characteristic of CLH. Five cases exhibited detectable clonal rearrangements of immunoglobulin genes. Furthermore, one of these five cases evolved into overt diffuse large cell lymphoma of B cell lineage during a 2-year follow-up of recurrent disease at the original cutaneous site. The immunoglobulin gene rearrangements of this lymphoma were identical to those of the prior CLH lesion. There was no evidence of detectable t(14;18) chromosomal translocations or clonal rearrangements of the beta gene of the T cell receptor in any case. It was concluded that CLH can be divided into two subsets based on the presence or absence of a clonal B cell population, and that overt lymphoma can arise from the former subset and contain the same B cell clone identified in the pre-existent CLH lesion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caro W. A., Helwig H. B. Cutaneous lymphoid hyperplasia. Cancer. 1969 Sep;24(3):487–502. doi: 10.1002/1097-0142(196909)24:3<487::aid-cncr2820240310>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Galili N., Sklar J. Detection of a second t(14;18) breakpoint cluster region in human follicular lymphomas. J Exp Med. 1986 Jul 1;164(1):315–320. doi: 10.1084/jem.164.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7439–7443. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Wood G. S., Warnke R., Chao J., Sklar J. Immunoglobulin gene rearrangements in hairy cell leukemia. Blood. 1984 Jul;64(1):99–104. [PubMed] [Google Scholar]
- Evans H. L., Winkelmann R. K., Banks P. M. Differential diagnosis of malignant and benign cutaneous lymphoid infiltrates: a study of 57 cases in which malignant lymphoma had been diagnosed or suspected in the skin. Cancer. 1979 Aug;44(2):699–717. doi: 10.1002/1097-0142(197908)44:2<699::aid-cncr2820440243>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Fishleder A., Tubbs R., Hesse B., Levine H. Uniform detection of immunoglobulin-gene rearrangement in benign lymphoepithelial lesions. N Engl J Med. 1987 Apr 30;316(18):1118–1121. doi: 10.1056/NEJM198704303161803. [DOI] [PubMed] [Google Scholar]
- Garcia C. F., Weiss L. M., Warnke R. A., Wood G. S. Cutaneous follicular lymphoma. Am J Surg Pathol. 1986 Jul;10(7):454–463. doi: 10.1097/00000478-198607000-00002. [DOI] [PubMed] [Google Scholar]
- Headington J. T., Roth M. S., Ginsburg D., Lichter A. S., Hyder D., Schnitzer B. T-cell receptor gene rearrangement in regressing atypical histiocytosis. Arch Dermatol. 1987 Sep;123(9):1183–1187. [PubMed] [Google Scholar]
- Knowles D. M., 2nd, Halper J. P., Jakobiec F. A. The immunologic characterization of 40 extranodal lymphoid infiltrates: usefulness in distinguishing between benign pseudolymphoma and malignant lymphoma. Cancer. 1982 Jun 1;49(11):2321–2335. doi: 10.1002/1097-0142(19820601)49:11<2321::aid-cncr2820491120>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Ravetch J. V., Poplack D. G., Waldmann T. A., Leder P. Developmental hierarchy of immunoglobulin gene rearrangements in human leukemic pre-B-cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7096–7100. doi: 10.1073/pnas.78.11.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle R. A. Monoclonal gammopathy of undetermined significance (MGUS): a review. Clin Haematol. 1982 Feb;11(1):123–150. [PubMed] [Google Scholar]
- LeBoit P. E., Beckstead J. H., Bond B., Epstein W. L., Frieden I. J., Parslow T. G. Granulomatous slack skin: clonal rearrangement of the T-cell receptor beta gene is evidence for the lymphoproliferative nature of a cutaneous elastolytic disorder. J Invest Dermatol. 1987 Aug;89(2):183–186. doi: 10.1111/1523-1747.ep12470557. [DOI] [PubMed] [Google Scholar]
- MacDonald D. M. Histopathological differentiation of benign and malignant cutaneous lymphocytic infiltrates. Br J Dermatol. 1982 Dec;107(6):715–718. doi: 10.1111/j.1365-2133.1982.tb00535.x. [DOI] [PubMed] [Google Scholar]
- McNally L., Jakobiec F. A., Knowles D. M., 2nd Clinical, morphologic, immunophenotypic, and molecular genetic analysis of bilateral ocular adnexal lymphoid neoplasms in 17 patients. Am J Ophthalmol. 1987 Apr 15;103(4):555–568. doi: 10.1016/s0002-9394(14)74280-1. [DOI] [PubMed] [Google Scholar]
- Neri A., Jakobiec F. A., Pelicci P. G., Dalla-Favera R., Knowles D. M., 2nd Immunoglobulin and T cell receptor beta chain gene rearrangement analysis of ocular adnexal lymphoid neoplasms: clinical and biologic implications. Blood. 1987 Nov;70(5):1519–1529. [PubMed] [Google Scholar]
- Payne C. M., Grogan T. M., Lynch P. J. An ultrastructural morphometric and immunohistochemical analysis of cutaneous lymphomas and benign lymphocytic infiltrates of skin. Useful criteria for diagnosis. Arch Dermatol. 1986 Oct;122(10):1139–1154. [PubMed] [Google Scholar]
- Pelicci P. G., Knowles D. M., 2nd, Dalla Favera R. Lymphoid tumors displaying rearrangements of both immunoglobulin and T cell receptor genes. J Exp Med. 1985 Sep 1;162(3):1015–1024. doi: 10.1084/jem.162.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Weiss L. M., Medeiros L. J., Wood G. S., Warnke R. A. Immunophenotypic criteria for the diagnosis of non-Hodgkin's lymphoma. Am J Pathol. 1987 Jul;128(1):181–201. [PMC free article] [PubMed] [Google Scholar]
- Ralfkiaer E., Saati T. A., Bosq J., Delsol G., Gatter K. C., Mason D. Y. Immunocytochemical characterisation of cutaneous lymphomas other than mycosis fungoides. J Clin Pathol. 1986 May;39(5):553–563. doi: 10.1136/jcp.39.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbitt M. L., Mackie R. M. An assessment of the diagnostic value of the monoclonal antibodies Leu 8, OKT9, OKT10 and Ki67 in cutaneous lymphocytic infiltrates. Br J Dermatol. 1986 Aug;115(2):151–158. doi: 10.1111/j.1365-2133.1986.tb05711.x. [DOI] [PubMed] [Google Scholar]
- Turner R. R., Egbert P., Warnke R. A. Lymphocytic infiltrates of the conjunctiva and orbit: immunohistochemical staining of 16 cases. Am J Clin Pathol. 1984 Apr;81(4):447–452. doi: 10.1093/ajcp/81.4.447. [DOI] [PubMed] [Google Scholar]
- Van Hale H. M., Winkelmann R. K. Nodular lymphoid disease of the head and neck: lymphocytoma cutis, benign lymphocytic infiltrate of Jessner, and their distinction from malignant lymphoma. J Am Acad Dermatol. 1985 Mar;12(3):455–461. doi: 10.1016/s0190-9622(85)70063-1. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Warnke R. A., Sklar J., Cleary M. L. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med. 1987 Nov 5;317(19):1185–1189. doi: 10.1056/NEJM198711053171904. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Wood G. S., Ellisen L. W., Reynolds T. C., Sklar J. Clonal T-cell populations in pityriasis lichenoides et varioliformis acuta (Mucha-Habermann disease). Am J Pathol. 1987 Mar;126(3):417–421. [PMC free article] [PubMed] [Google Scholar]
- Weiss L. M., Wood G. S., Trela M., Warnke R. A., Sklar J. Clonal T-cell populations in lymphomatoid papulosis. Evidence of a lymphoproliferative origin for a clinically benign disease. N Engl J Med. 1986 Aug 21;315(8):475–479. doi: 10.1056/NEJM198608213150802. [DOI] [PubMed] [Google Scholar]
- Willemze R., de Graaff-Reitsma C. B., van Vloten W. A., Meijer C. J. The cell population of cutaneous B-cell lymphomas. Br J Dermatol. 1983 Apr;108(4):395–409. doi: 10.1111/j.1365-2133.1983.tb04592.x. [DOI] [PubMed] [Google Scholar]
- Williams M. E., Innes D. J., Jr, Borowitz M. J., Lovell M. A., Swerdlow S. H., Hurtubise P. E., Brynes R. K., Chan W. C., Byrne G. E., Jr, Whitcomb C. C. Immunoglobulin and T cell receptor gene rearrangements in human lymphoma and leukemia. Blood. 1987 Jan;69(1):79–86. [PubMed] [Google Scholar]
- Wirt D. P., Grogan T. M., Jolley C. S., Rangel C. S., Payne C. M., Hansen R. C., Lynch P. J., Schuchardt M. The immunoarchitecture of cutaneous pseudolymphoma. Hum Pathol. 1985 May;16(5):492–510. doi: 10.1016/s0046-8177(85)80089-7. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Burke J. S., Horning S., Doggett R. S., Levy R., Warnke R. A. The immunologic and clinicopathologic heterogeneity of cutaneous lymphomas other than mycosis fungoides. Blood. 1983 Aug;62(2):464–472. [PubMed] [Google Scholar]
- Wood G. S., Warnke R. A. The immunologic phenotyping of bone marrow biopsies and aspirates: frozen section techniques. Blood. 1982 May;59(5):913–922. [PubMed] [Google Scholar]
- Wood G. S., Weiss L. M., Hu C. H., Abel E. A., Hoppe R. T., Warnke R. A., Sklar J. T-cell antigen deficiencies and clonal rearrangements of T-cell receptor genes in pagetoid reticulosis (Woringer-Kolopp disease). N Engl J Med. 1988 Jan 21;318(3):164–167. doi: 10.1056/NEJM198801213180307. [DOI] [PubMed] [Google Scholar]