Abstract
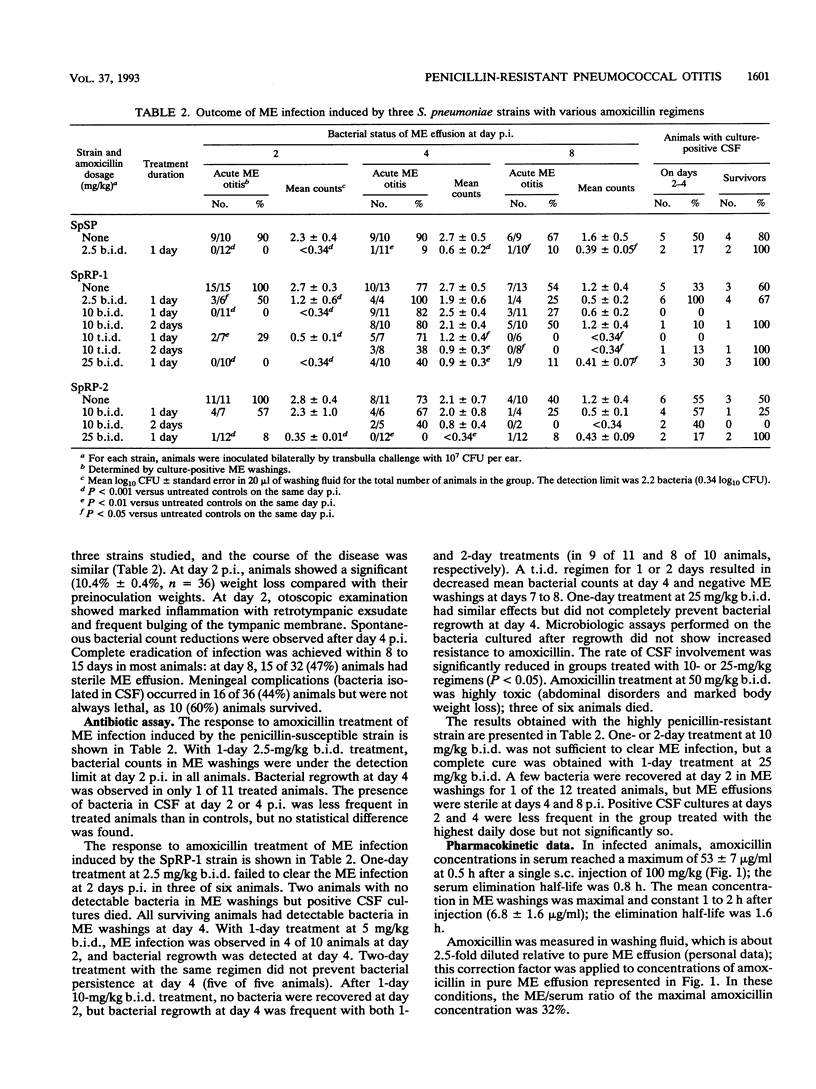
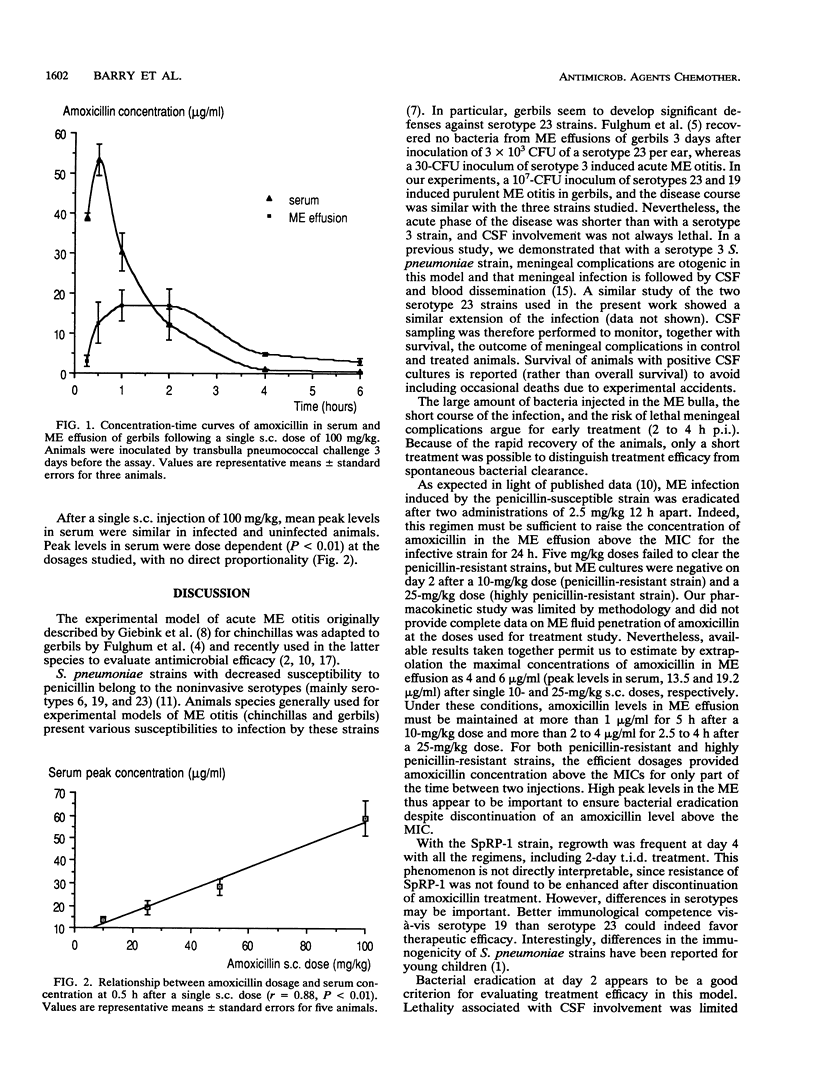
A gerbil model of acute middle ear otitis was used to evaluate the efficacy of increased dosages of amoxicillin in eradicating infection induced by penicillin-resistant Streptococcus pneumoniae. Three different strains were used: (i) a serotype 23 penicillin-susceptible strain; (ii) a serotype 23 penicillin-resistant strain (MIC of penicillin, 2 micrograms/ml); and (iii) a serotype 19 highly penicillin-resistant strain (MIC of penicillin, 4 to 8 micrograms/ml). Animals were inoculated bilaterally with 10(7) CFU per ear by transbulla challenge and treated 2 to 4 h postinfection by amoxicillin administrated subcutaneously. The course of the disease was monitored bacteriologically on days 2, 4, and 8 postinfection. The three strains had a similar pathogenicity in untreated animals in terms of the duration of the disease, bacterial counts in middle ear (ME) fluid, and systemic complications. Infection due to the penicillin-susceptible strain was cured after two injections of 2.5 mg/kg of body weight. No bacteria were recovered at day 2 after two injections at 10 and 25 mg/kg with the penicillin-resistant and highly penicillin-resistant strains, respectively. Under these experimental conditions, increased does of amoxicillin consistent with MICs were able to clear ME infection. Pharmacokinetic parameters of amoxicillin in serum and ME fluid were within the clinical range at the doses used in the study.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruyn G. A., Zegers B. J., van Furth R. Mechanisms of host defense against infection with Streptococcus pneumoniae. Clin Infect Dis. 1992 Jan;14(1):251–262. doi: 10.1093/clinids/14.1.251. [DOI] [PubMed] [Google Scholar]
- Ford K. L., Mason E. O., Jr, Kaplan S. L., Lamberth L. B., Tillman J. Factors associated with middle ear isolates of Streptococcus pneumoniae resistant to penicillin in a children's hospital. J Pediatr. 1991 Dec;119(6):941–944. doi: 10.1016/s0022-3476(05)83050-1. [DOI] [PubMed] [Google Scholar]
- Fulghum R. S., Brinn J. E., Smith A. M., Daniel H. J., 3rd, Loesche P. J. Experimental otitis media in gerbils and chinchillas with Streptococcus pneumoniae, Haemophilus influenzae, and other aerobic and anaerobic bacteria. Infect Immun. 1982 May;36(2):802–810. doi: 10.1128/iai.36.2.802-810.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulghum R. S., Hoogmoed R. P., Brinn J. E., Smith A. M. Experimental pneumococcal otitis media: longitudinal studies in the gerbil model. Int J Pediatr Otorhinolaryngol. 1985 Oct;10(1):9–20. doi: 10.1016/s0165-5876(85)80052-5. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Berzins I. K., Quie P. G. Animal models for studying pneumococcal otitis media and pneumococcal vaccine efficacy. Ann Otol Rhinol Laryngol Suppl. 1980 May-Jun;89(3 Pt 2):339–343. doi: 10.1177/00034894800890s380. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Payne E. E., Mills E. L., Juhn S. K., Quie P. G. Experimental otitis media due to Streptococcus pneumoniae: immunopathogenic response in the chinchilla. J Infect Dis. 1976 Dec;134(6):595–604. doi: 10.1093/infdis/134.6.595. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Schiffman G., Petty K., Quie P. G. Modification of otitis media following vaccination with the capsular polysaccharide of Streptococcus pneumoniae in chinchillas. J Infect Dis. 1978 Oct;138(4):480–487. doi: 10.1093/infdis/138.4.480. [DOI] [PubMed] [Google Scholar]
- Girard A. E., Girard D., English A. R., Gootz T. D., Cimochowski C. R., Faiella J. A., Haskell S. L., Retsema J. A. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987 Dec;31(12):1948–1954. doi: 10.1128/aac.31.12.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugman K. P. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990 Apr;3(2):171–196. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause P. J., Owens N. J., Nightingale C. H., Klimek J. J., Lehmann W. B., Quintiliani R. Penetration of amoxicillin, cefaclor, erythromycin-sulfisoxazole, and trimethoprim-sulfamethoxazole into the middle ear fluid of patients with chronic serous otitis media. J Infect Dis. 1982 Jun;145(6):815–821. doi: 10.1093/infdis/145.6.815. [DOI] [PubMed] [Google Scholar]
- Marton A., Gulyas M., Munoz R., Tomasz A. Extremely high incidence of antibiotic resistance in clinical isolates of Streptococcus pneumoniae in Hungary. J Infect Dis. 1991 Mar;163(3):542–548. doi: 10.1093/infdis/163.3.542. [DOI] [PubMed] [Google Scholar]
- Michel J., Dickman D., Greenberg Z., Bergner-Rabinowitz S. Serotype distribution of penicillin-resistant pneumococci and their susceptibilities to seven antimicrobial agents. Antimicrob Agents Chemother. 1983 Mar;23(3):397–401. doi: 10.1128/aac.23.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R. N., Hardy D. J., Chu D. T., Shipkowitz N. L., Clement J. J. Activity of temafloxacin against respiratory pathogens. Antimicrob Agents Chemother. 1991 Mar;35(3):423–429. doi: 10.1128/aac.35.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]



