Abstract
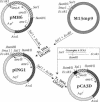
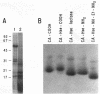
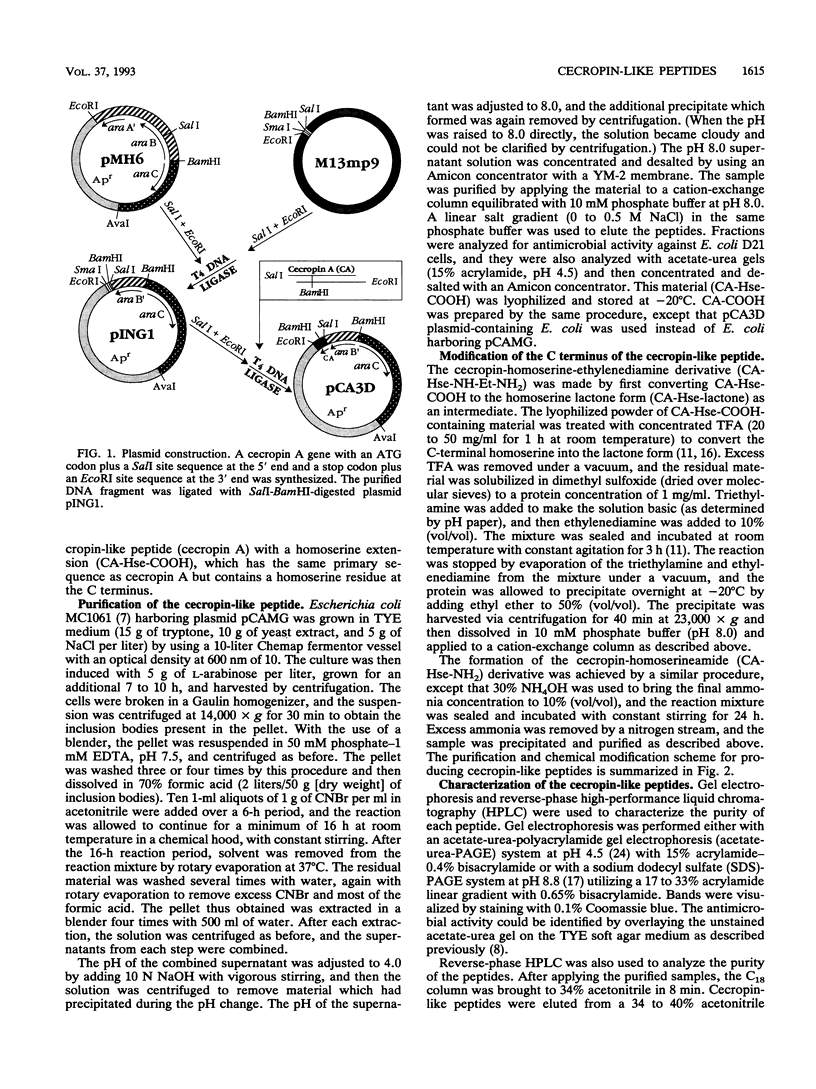
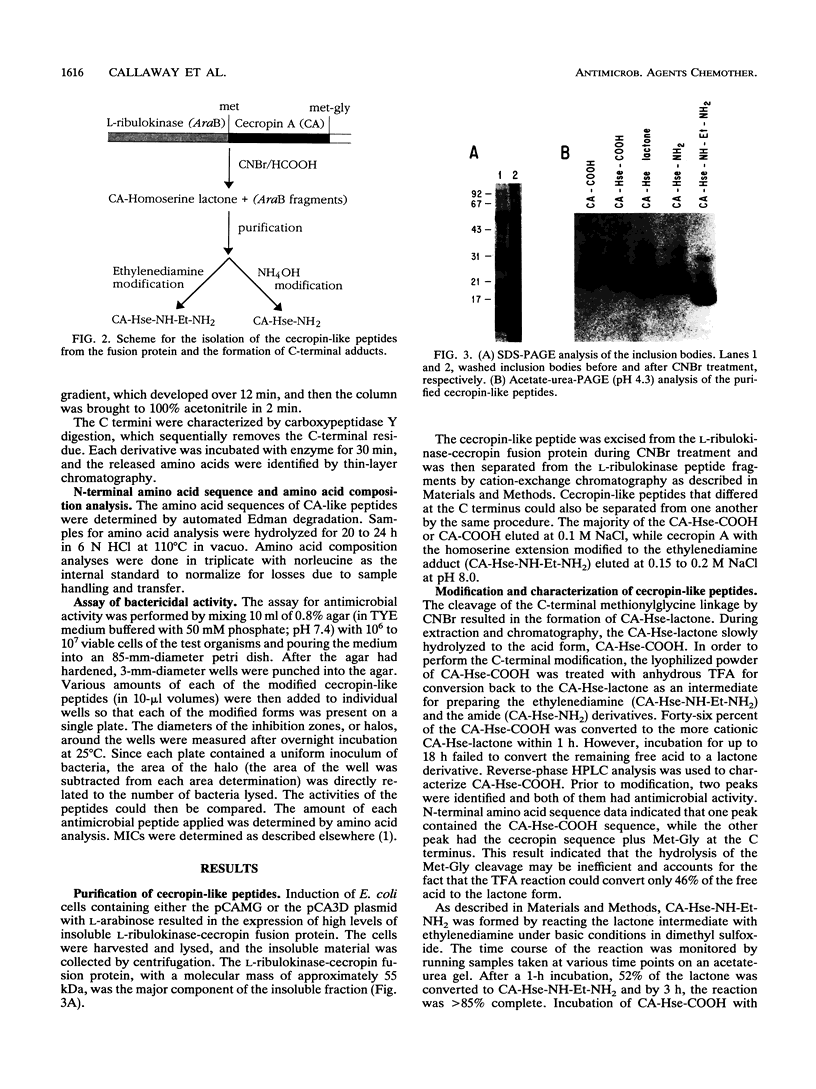
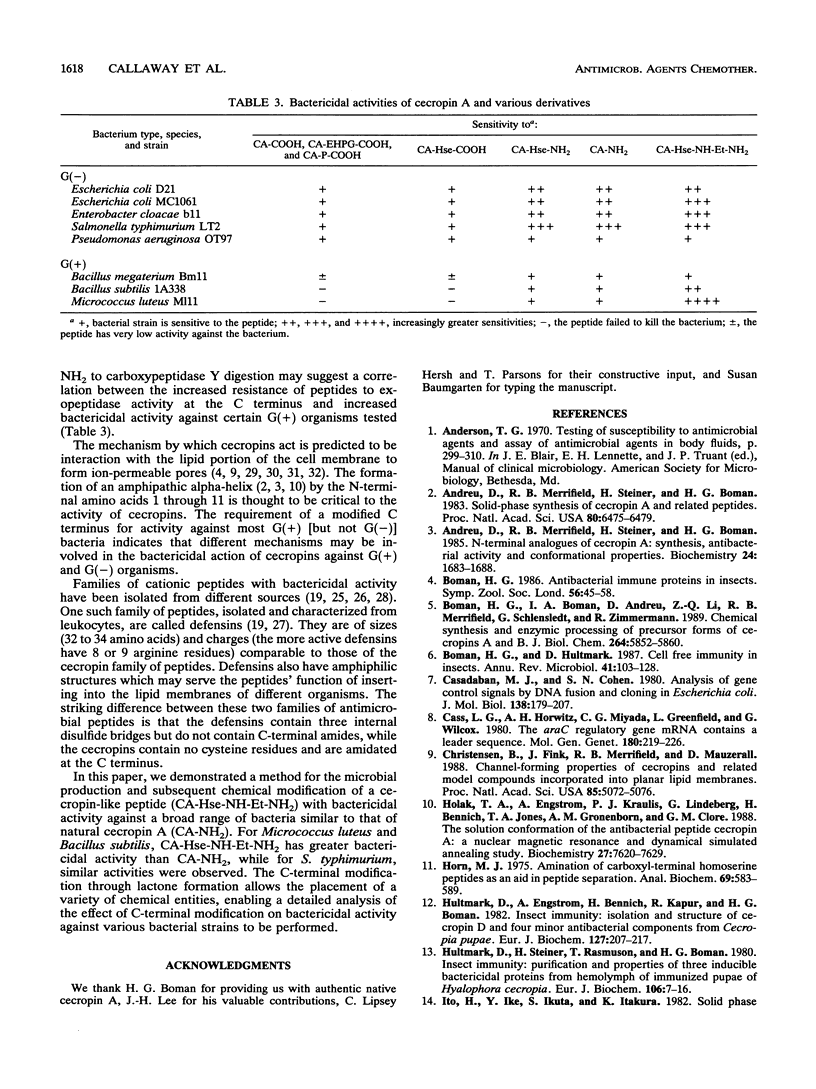
Cecropin A is a naturally occurring peptide with bactericidal activity against gram-negative and gram-positive bacteria. Production of large quantities of bactericidal peptides that are similar in structure and activity to cecropin A has been achieved by combining recombinant DNA techniques and techniques and chemical modification. Expression of the bactericidal peptide in Escherichia coli was accomplished through the formation of a fusion protein. The 5' end of the L-ribulokinase gene was fused to a single copy of a synthetic gene encoding cecropin A. A methionine codon was engineered between the two genes, and a methionylglycine extension was introduced at the C terminus of cecropin A. Cyanogen bromide treatment of the fusion protein yielded cecropin A with a C-terminal homoserine. The recombinant cecropin A with a homoserine at the C terminus did not kill most gram-positive bacteria tested. However, recombinant cecropin A with a chemically modified C terminus has antimicrobial activity similar to that of cecropin produced by cecropia pupae.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreu D., Merrifield R. B., Steiner H., Boman H. G. N-terminal analogues of cecropin A: synthesis, antibacterial activity, and conformational properties. Biochemistry. 1985 Mar 26;24(7):1683–1688. doi: 10.1021/bi00328a017. [DOI] [PubMed] [Google Scholar]
- Andreu D., Merrifield R. B., Steiner H., Boman H. G. Solid-phase synthesis of cecropin A and related peptides. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6475–6479. doi: 10.1073/pnas.80.21.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. C., Boman I. A., Andreu D., Li Z. Q., Merrifield R. B., Schlenstedt G., Zimmermann R. Chemical synthesis and enzymic processing of precursor forms of cecropins A and B. J Biol Chem. 1989 Apr 5;264(10):5852–5860. [PubMed] [Google Scholar]
- Boman H. G., Hultmark D. Cell-free immunity in insects. Annu Rev Microbiol. 1987;41:103–126. doi: 10.1146/annurev.mi.41.100187.000535. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Cass L. G., Horwitz A. H., Miyada C. G., Greenfield L., Wilcox G. The araC regulatory gene mRNA contains a leader sequence. Mol Gen Genet. 1980;180(1):219–226. doi: 10.1007/BF00267373. [DOI] [PubMed] [Google Scholar]
- Christensen B., Fink J., Merrifield R. B., Mauzerall D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5072–5076. doi: 10.1073/pnas.85.14.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holak T. A., Engström A., Kraulis P. J., Lindeberg G., Bennich H., Jones T. A., Gronenborn A. M., Clore G. M. The solution conformation of the antibacterial peptide cecropin A: a nuclear magnetic resonance and dynamical simulated annealing study. Biochemistry. 1988 Oct 4;27(20):7620–7629. doi: 10.1021/bi00420a008. [DOI] [PubMed] [Google Scholar]
- Horn M. J. Amination of carboxyl-terminal homoserine peptides as an aid in peptide separation. Anal Biochem. 1975 Dec;69(2):583–589. doi: 10.1016/0003-2697(75)90163-3. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Engström A., Bennich H., Kapur R., Boman H. G. Insect immunity: isolation and structure of cecropin D and four minor antibacterial components from Cecropia pupae. Eur J Biochem. 1982 Sep;127(1):207–217. doi: 10.1111/j.1432-1033.1982.tb06857.x. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Steiner H., Rasmuson T., Boman H. G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem. 1980 May;106(1):7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- Ito H., Ike Y., Ikuta S., Itakura K. Solid phase synthesis of polynucleotides. VI. Further studies on polystyrene copolymers for the solid support. Nucleic Acids Res. 1982 Mar 11;10(5):1755–1769. doi: 10.1093/nar/10.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S., Lee J. H., Ray D. S. High-level expression of M13 gene II protein from an inducible polycistronic messenger RNA. Gene. 1985;34(2-3):137–145. doi: 10.1016/0378-1119(85)90121-0. [DOI] [PubMed] [Google Scholar]
- Kempe T., Kent S. B., Chow F., Peterson S. M., Sundquist W. I., L'Italien J. J., Harbrecht D., Plunkett D., DeLorbe W. J. Multiple-copy genes: production and modification of monomeric peptides from large multimeric fusion proteins. Gene. 1985;39(2-3):239–245. doi: 10.1016/0378-1119(85)90318-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. Y., Boman A., Sun C. X., Andersson M., Jörnvall H., Mutt V., Boman H. G. Antibacterial peptides from pig intestine: isolation of a mammalian cecropin. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9159–9162. doi: 10.1073/pnas.86.23.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T. Antimicrobial polypeptides of human neutrophils. Blood. 1990 Dec 1;76(11):2169–2181. [PubMed] [Google Scholar]
- Li Z. Q., Merrifield R. B., Boman I. A., Boman H. G. Effects on electrophoretic mobility and antibacterial spectrum of removal of two residues from synthetic sarcotoxin IA and addition of the same residues to cecropin B. FEBS Lett. 1988 Apr 25;231(2):299–302. doi: 10.1016/0014-5793(88)80837-8. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B., Vizioli L. D., Boman H. G. Synthesis of the antibacterial peptide cecropin A (1-33). Biochemistry. 1982 Sep 28;21(20):5020–5031. doi: 10.1021/bi00263a028. [DOI] [PubMed] [Google Scholar]
- Miyada C. G., Soberón X., Itakura K., Wilcox G. The use of synthetic oligodeoxyribonucleotides to produce specific deletions in the araBAD promoter of Escherichia coli B/r. Gene. 1982 Feb;17(2):167–177. doi: 10.1016/0378-1119(82)90070-1. [DOI] [PubMed] [Google Scholar]
- Qu Z., Steiner H., Engström A., Bennich H., Boman H. G. Insect immunity: isolation and structure of cecropins B and D from pupae of the Chinese oak silk moth, Antheraea pernyi. Eur J Biochem. 1982 Sep;127(1):219–224. doi: 10.1111/j.1432-1033.1982.tb06858.x. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Selsted M. E., Brown D. M., DeLange R. J., Harwig S. S., Lehrer R. I. Primary structures of six antimicrobial peptides of rabbit peritoneal neutrophils. J Biol Chem. 1985 Apr 25;260(8):4579–4584. [PubMed] [Google Scholar]
- Selsted M. E., Brown D. M., DeLange R. J., Lehrer R. I. Primary structures of MCP-1 and MCP-2, natural peptide antibiotics of rabbit lung macrophages. J Biol Chem. 1983 Dec 10;258(23):14485–14489. [PubMed] [Google Scholar]
- Selsted M. E., Harwig S. S., Ganz T., Schilling J. W., Lehrer R. I. Primary structures of three human neutrophil defensins. J Clin Invest. 1985 Oct;76(4):1436–1439. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Szklarek D., Lehrer R. I. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infect Immun. 1984 Jul;45(1):150–154. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H., Andreu D., Merrifield R. B. Binding and action of cecropin and cecropin analogues: antibacterial peptides from insects. Biochim Biophys Acta. 1988 Apr 7;939(2):260–266. doi: 10.1016/0005-2736(88)90069-7. [DOI] [PubMed] [Google Scholar]
- Steiner H., Hultmark D., Engström A., Bennich H., Boman H. G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981 Jul 16;292(5820):246–248. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- Steiner H. Secondary structure of the cecropins: antibacterial peptides from the moth Hyalophora cecropia. FEBS Lett. 1982 Jan 25;137(2):283–287. doi: 10.1016/0014-5793(82)80368-2. [DOI] [PubMed] [Google Scholar]
- Wade D., Boman A., Wåhlin B., Drain C. M., Andreu D., Boman H. G., Merrifield R. B. All-D amino acid-containing channel-forming antibiotic peptides. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4761–4765. doi: 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]