Abstract
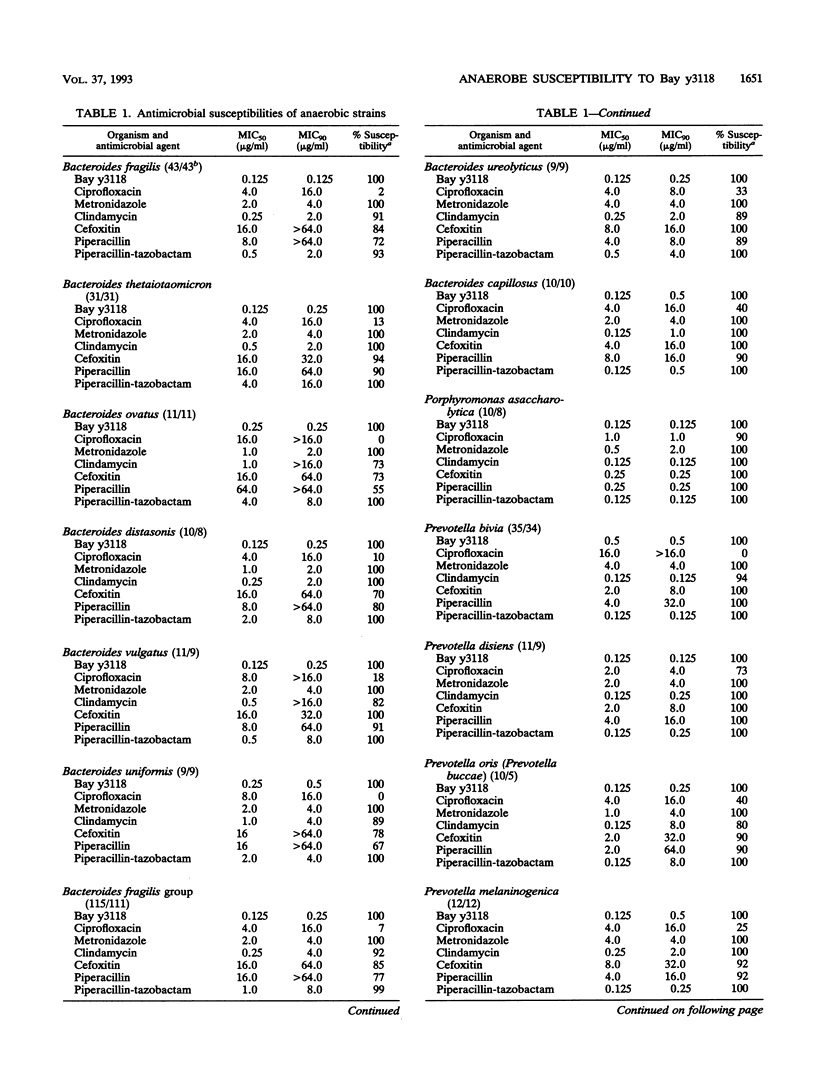
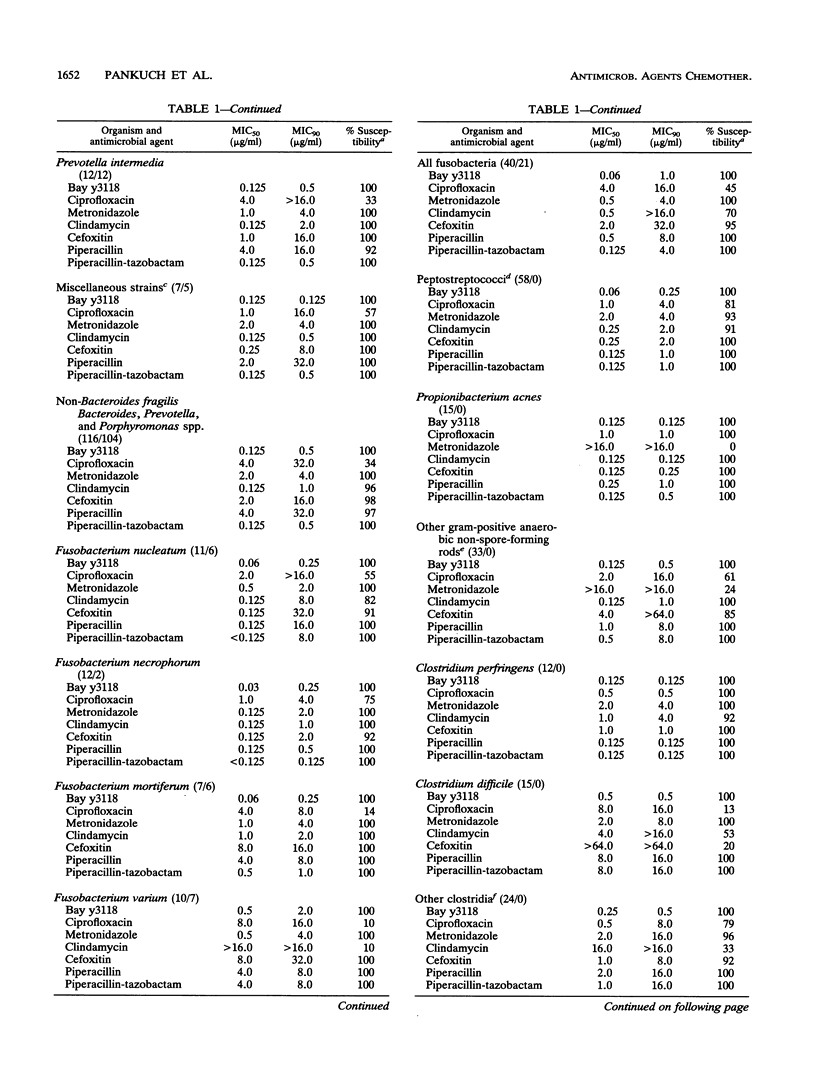
The susceptibilities of 428 gram-negative and gram-positive anaerobes (including selected cefoxitin-resistant strains) to Bay y3118 (a new fluoroquinolone), ciprofloxacin, clindamycin, metronidazole, cefoxitin, piperacillin, and piperacillin-tazobactam were tested. Organisms comprised 115 Bacteroides fragilis group, 116 non-B. fragilis Bacteroides, Prevotella, and Porphyromonas spp., 40 fusobacteria, 58 peptostreptococci, 48 gram-positive non-spore-forming rods, and 51 clostridia. beta-Lactamase production was demonstrated in 87% of the gram-negative rods but in none of the gram-positive organisms. Overall, Bay y3118 was the most active agent, with all organisms inhibited at an MIC of < or = 2.0 micrograms/ml (MICs for 50% [MIC50] and 90% [MIC90] of strains tested, 0.125 and 0.5 microgram/ml, respectively). By contrast, ciprofloxacin was much less active, with only 42% of strains susceptible at a breakpoint of 2.0 micrograms/ml (MIC50, 4.0 micrograms/ml; MIC90, 16.0 micrograms/ml). Metronidazole was active against all gram-negative rods, but 7% of peptostreptococci, 83% of gram-positive non-spore-forming rods, and 4% of non-Clostridium perfringens, non-Clostridium difficile clostridia were resistant to this agent (MICs, > 16.0 micrograms/ml). Clindamycin was active against 94% of Bacteroides, Prevotella, and Porphyromonas spp., 91% of peptostreptococci, and 100% of gram-positive non-spore-forming rods, but was active against only 70% of fusobacteria and 53% of clostridia. Cefoxitin was active against > or = 90% of all groups except the B. fragilis group and non-Propionibacterium acnes gram-positive non-spore-forming rods (both 85%) and C. difficile (20%). Significant enhancement of piperacillin by tazobactam was seen in all beta-lactamase-positive strains (99% susceptible; MIC90, 8.0 micrograms/ml), and all beta-lactamase-negative strains were susceptible to piperacillin (MIC90, 8.0 micrograms/ml). Clinical studies are required to delineate the role of Bay y3118 in the treatment of anaerobic infections.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum P. C., Jacobs M. R., Spangler S. K., Yamabe S. Comparative activity of beta-lactamase inhibitors YTR 830, clavulanate, and sulbactam combined with beta-lactams against beta-lactamase-producing anaerobes. Antimicrob Agents Chemother. 1986 Nov;30(5):789–791. doi: 10.1128/aac.30.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum P. C., Philippon A., Jacobs M. R., Spangler S. K., Gutmann L. Characterization of beta-lactamases from non-Bacteroides fragilis group Bacteroides spp. belonging to seven species and their role in beta-lactam resistance. Antimicrob Agents Chemother. 1990 Nov;34(11):2169–2176. doi: 10.1128/aac.34.11.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum P. C., Spangler S. K., Jacobs M. R. Beta-lactamase production and susceptibilities to amoxicillin, amoxicillin-clavulanate, ticarcillin, ticarcillin-clavulanate, cefoxitin, imipenem, and metronidazole of 320 non-Bacteroides fragilis Bacteroides isolates and 129 fusobacteria from 28 U.S. centers. Antimicrob Agents Chemother. 1990 Aug;34(8):1546–1550. doi: 10.1128/aac.34.8.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum P. C., Spangler S. K., Jacobs M. R. Evaluation of two methods for rapid testing for beta-lactamase production in Bacteroides and Fusobacterium. Eur J Clin Microbiol Infect Dis. 1990 Jan;9(1):47–50. doi: 10.1007/BF01969535. [DOI] [PubMed] [Google Scholar]
- Appelbaum P. C., Spangler S. K., Jacobs M. R. Susceptibilities of 394 Bacteroides fragilis, non-B. fragilis group Bacteroides species, and Fusobacterium species to newer antimicrobial agents. Antimicrob Agents Chemother. 1991 Jun;35(6):1214–1218. doi: 10.1128/aac.35.6.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum P. C., Spangler S. K., Shiman R., Jacobs M. R. Susceptibilities of 540 anaerobic gram-negative bacilli to amoxicillin, amoxicillin-BRL 42715, amoxicillin-clavulanate, temafloxacin, and clindamycin. Antimicrob Agents Chemother. 1992 May;36(5):1140–1143. doi: 10.1128/aac.36.5.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandoh K., Ueno K., Watanabe K., Kato N. Susceptibility patterns and resistance to imipenem in the Bacteroides fragilis group species in Japan: a 4-year study. Clin Infect Dis. 1993 Jun;16 (Suppl 4):S382–S386. doi: 10.1093/clinids/16.supplement_4.s382. [DOI] [PubMed] [Google Scholar]
- Chow A. W., Patten V., Guze L. B. Susceptibility of anaerobic bacteria to metronidazole: relative resistance of non-spore-forming gram-positive baccilli. J Infect Dis. 1975 Feb;131(2):182–185. doi: 10.1093/infdis/131.2.182. [DOI] [PubMed] [Google Scholar]
- Edson R. S., Rosenblatt J. E., Lee D. T., McVey E. A., 3rd Recent experience with antimicrobial susceptibility of anaerobic bacteria: increasing resistance to penicillin. Mayo Clin Proc. 1982 Dec;57(12):737–741. [PubMed] [Google Scholar]
- George W. L., Kirby B. D., Sutter V. L., Citron D. M., Finegold S. M. Gram-negative anaerobic bacilli: Their role in infection and patterns of susceptibility to antimicrobial agents. II. Little-known Fusobacterium species and miscellaneous genera. Rev Infect Dis. 1981 May-Jun;3(3):599–626. doi: 10.1093/clinids/3.3.599. [DOI] [PubMed] [Google Scholar]
- Goldstein E. J., Citron D. M. Comparative activity of ciprofloxacin, ofloxacin, sparfloxacin, temafloxacin, CI-960, CI-990, and WIN 57273 against anaerobic bacteria. Antimicrob Agents Chemother. 1992 May;36(5):1158–1162. doi: 10.1128/aac.36.5.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E. J., Citron D. M. Comparative activity of the quinolones against anaerobic bacteria isolated at community hospitals. Antimicrob Agents Chemother. 1985 Apr;27(4):657–659. doi: 10.1128/aac.27.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M. R., Spangler S. K., Appelbaum P. C. Beta-lactamase production, beta-lactam sensitivity and resistance to synergy with clavulanate of 737 Bacteroides fragilis group organisms from thirty-three US centres. J Antimicrob Chemother. 1990 Sep;26(3):361–370. doi: 10.1093/jac/26.3.361. [DOI] [PubMed] [Google Scholar]
- Nord C. E. Mechanisms of beta-lactam resistance in anaerobic bacteria. Rev Infect Dis. 1986 Nov-Dec;8 (Suppl 5):S543–S548. doi: 10.1093/clinids/8.supplement_5.s543. [DOI] [PubMed] [Google Scholar]
- Rao D. S., Parfitt A. M., Villanueva A. R., Dorman P. J., Kleerekoper M. Hypophosphatemic osteomalacia and adult Fanconi syndrome due to light-chain nephropathy. Another form of oncogenous osteomalacia. Am J Med. 1987 Feb;82(2):333–338. doi: 10.1016/0002-9343(87)90081-7. [DOI] [PubMed] [Google Scholar]
- Rolfe R. D., Finegold S. M. Comparative in vitro activity of ceftriaxone against anaerobic bacteria. Antimicrob Agents Chemother. 1982 Aug;22(2):338–341. doi: 10.1128/aac.22.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spera R. V., Jr, Kaplan M. H., Allen S. L. Clostridium sordellii bacteremia: case report and review. Clin Infect Dis. 1992 Dec;15(6):950–954. doi: 10.1093/clind/15.6.950. [DOI] [PubMed] [Google Scholar]


