Abstract
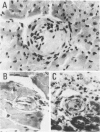
To determine whether or not male (NZWXBXSB)F1 [WXB)F1) mice exhibiting a lupus-like syndrome died of acute myocardial infarction (MI), and whether acute MI is directly related to small coronary artery disease, acute and old MIs were examined histologically in 55 dead (WXB)F1 male mice and 30 age-matched, surviving (WXB)F1 male mice used as a control group. In each heart from the 15 dead mice with MI and the five surviving mice without MI, 300 to 400 5-microns-thick serial sections were made; every fourth section was stained. Acute MI was found in 35 (64%) dead mice and in one (3%) survivors, whereas old MI was found in 50 (91%) dead mice and 17 (57%) survivors: a significant difference between the dead and surviving mice. The MIs were numerous, scattered, and small in most mice. Quantitative analysis revealed that the percentage of acute MI and old MI in the left ventricular (LV) wall was 6% +/- 11% and 3% +/- 3% in the dead group, and 0.4% and 2% +/- 3% in the control group. This indicated that recurrent acute MI is a major factor in the death of the mice. Although all the epicardial major coronary arteries of the (WXB)F1 male mice were intact, significant stenoses were noted in the intramyocardial small arteries. The serial sections in the 15 dead mice with MI revealed 1) segmental occlusive thrombi in the infarct-related small coronary artery in 14 of the 20 foci of acute anemic MIs, two of the 18 foci of acute hemorrhagic MIs, and four of the 58 foci of old MIs; and 2) segmental intimal thickenings in the infarct-related small artery in six of the 20 foci of acute anemic MIs, two of the 18 foci of acute hemorrhagic MIs, and 56 of the 58 foci of old MIs. There was no evidence of small coronary artery disease in the surviving mice without MI. The thrombus would result in thickened intima as MI progresses from the acute to the old stage. Because it was established that acute MI of hemorrhagic type follows reperfusion after transient occlusion of the coronary artery, hemorrhagic acute MI with rare incidence of thrombi in this mouse suggests that thrombolysis occurs after occlusion due to thrombus formation. Thus, the pathogenesis of multiple MIs is occlusive thrombi, recanalization in small coronary arteries or both. Some of the mice had dilated cardiomyopathy (DCM)-like features (marked LV dilatation).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angles-Cano E., Sultan Y., Clauvel J. P. Predisposing factors to thrombosis in systemic lupus erythematosus: possible relation to endothelial cell damage. J Lab Clin Med. 1979 Aug;94(2):312–323. [PubMed] [Google Scholar]
- Asherson R. A., Harris N., Gharavi A., Hughes G. R. Myocardial infarction in systemic lupus erythematosus and "lupus-like" disease. Arthritis Rheum. 1986 Oct;29(10):1292–1293. doi: 10.1002/art.1780291019. [DOI] [PubMed] [Google Scholar]
- Berden J. H., Hang L., McConahey P. J., Dixon F. J. Analysis of vascular lesions in murine SLE. I. Association with serologic abnormalities. J Immunol. 1983 Apr;130(4):1699–1705. [PubMed] [Google Scholar]
- Bidani A. K., Roberts J. L., Schwartz M. M., Lewis E. J. Immunopathology of cardiac lesions in fatal systemic lupus erythematosus. Am J Med. 1980 Dec;69(6):849–858. doi: 10.1016/s0002-9343(80)80010-6. [DOI] [PubMed] [Google Scholar]
- Bresnahan G. F., Roberts R., Shell W. E., Ross J., Jr, Sobel B. E. Deleterious effects due to hemorrhage after myocardial reperfusion. Am J Cardiol. 1974 Jan;33(1):82–86. doi: 10.1016/0002-9149(74)90742-5. [DOI] [PubMed] [Google Scholar]
- Capone R. J., Most A. S. Myocardial hemorrhage after coronary reperfusion in pigs. Am J Cardiol. 1978 Feb;41(2):259–266. doi: 10.1016/0002-9149(78)90164-9. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Sonnenblick E. H. Hypothesis: is congestive cardiomyopathy caused by a hyperreactive myocardial microcirculation (microvascular spasm)? Am J Cardiol. 1982 Nov;50(5):1149–1152. doi: 10.1016/0002-9149(82)90435-0. [DOI] [PubMed] [Google Scholar]
- Fujiwara H., Ashraf M., Sato S., Millard R. W. Transmural cellular damage and blood flow distribution in early ischemia in pig hearts. Circ Res. 1982 Dec;51(6):683–693. doi: 10.1161/01.res.51.6.683. [DOI] [PubMed] [Google Scholar]
- Fujiwara H., Fujiwara T., Tanaka M., Onodera T., Miyazaki S., Wu D. J., Matsuda M., Sasayama S., Kawai C. Detection of early myocardial infarction in formalin-fixed, paraffin-embedded tissue. Am J Cardiovasc Pathol. 1988;2(1):57–61. [PubMed] [Google Scholar]
- Fujiwara H., Onodera T., Tanaka M., Fujiwara T., Wu D. J., Kawai C., Hamashima Y. A clinicopathologic study of patients with hemorrhagic myocardial infarction treated with selective coronary thrombolysis with urokinase. Circulation. 1986 Apr;73(4):749–757. doi: 10.1161/01.cir.73.4.749. [DOI] [PubMed] [Google Scholar]
- Fujiwara H., Onodera T., Tanaka M., Miyazaki S., Wu D. J., Matsuda M., Kawamura A., Ishida M., Takemura G., Fujiwara Y. Acceleration of cell necrosis following reperfusion after ischemia in the pig heart without collateral circulation. Am J Cardiol. 1989 Mar 7;63(10):14E–18E. doi: 10.1016/0002-9149(89)90224-5. [DOI] [PubMed] [Google Scholar]
- Hamsten A., Norberg R., Björkholm M., de Faire U., Holm G. Antibodies to cardiolipin in young survivors of myocardial infarction: an association with recurrent cardiovascular events. Lancet. 1986 Jan 18;1(8473):113–116. doi: 10.1016/s0140-6736(86)92258-0. [DOI] [PubMed] [Google Scholar]
- Hang L. M., Izui S., Dixon F. J. (NZW x BXSB)F1 hybrid. A model of acute lupus and coronary vascular disease with myocardial infarction. J Exp Med. 1981 Jul 1;154(1):216–221. doi: 10.1084/jem.154.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang L., Stephen-Larson P. M., Henry J. P., Dixon F. J. Transfer of renovascular hypertension and coronary heart disease by lymphoid cells from SLE-prone mice. Am J Pathol. 1984 Apr;115(1):42–46. [PMC free article] [PubMed] [Google Scholar]
- Hang L., Stephens-Larson P., Henry J. P., Dixon F. J. The role of hypertension in the vascular disease and myocardial infarcts associated with murine systemic lupus erythematosus. Arthritis Rheum. 1983 Nov;26(11):1340–1345. doi: 10.1002/art.1780261106. [DOI] [PubMed] [Google Scholar]
- Higginson L. A., Beanlands D. S., Nair R. C., Temple V., Sheldrick K. The time course and characterization of myocardial hemorrhage after coronary reperfusion in the anesthetized dog. Circulation. 1983 May;67(5):1024–1031. doi: 10.1161/01.cir.67.5.1024. [DOI] [PubMed] [Google Scholar]
- Hudgins C. C., Steinberg R. T., Klinman D. M., Reeves M. J., Steinberg A. D. Studies of consomic mice bearing the Y chromosome of the BXSB mouse. J Immunol. 1985 Jun;134(6):3849–3854. [PubMed] [Google Scholar]
- Lund D. D., Tomanek R. J. Myocardial morphology in spontaneously hypertensive and aortic-constricted rats. Am J Anat. 1978 Jun;152(2):141–151. doi: 10.1002/aja.1001520202. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Epstein S. E., Roberts W. C. Hypertrophic cardiomyopathy and transmural myocardial infarction without significant atherosclerosis of the extramural coronary arteries. Am J Cardiol. 1979 Jun;43(6):1086–1102. doi: 10.1016/0002-9149(79)90139-5. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Fujiwara H., Onodera T., Tanaka M., Wu D. J., Fujiwara T., Hamashima Y., Kawai C. Quantitative analysis of infarct size, contraction band necrosis, and coagulation necrosis in human autopsied hearts with acute myocardial infarction after treatment with selective intracoronary thrombolysis. Circulation. 1987 Nov;76(5):981–989. doi: 10.1161/01.cir.76.5.981. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Fujiwara H., Onodera T., Kihara Y., Matsuda M., Wu D. J., Nakamura Y., Kumada T., Sasayama S., Kawai C. Quantitative analysis of contraction band and coagulation necrosis after ischemia and reperfusion in the porcine heart. Circulation. 1987 May;75(5):1074–1082. doi: 10.1161/01.cir.75.5.1074. [DOI] [PubMed] [Google Scholar]
- Mosseri M., Yarom R., Gotsman M. S., Hasin Y. Histologic evidence for small-vessel coronary artery disease in patients with angina pectoris and patent large coronary arteries. Circulation. 1986 Nov;74(5):964–972. doi: 10.1161/01.cir.74.5.964. [DOI] [PubMed] [Google Scholar]
- Mueh J. R., Herbst K. D., Rapaport S. I. Thrombosis in patients with the lupus anticoagulant. Ann Intern Med. 1980 Feb;92(2 Pt 1):156–159. doi: 10.7326/0003-4819-92-2-156. [DOI] [PubMed] [Google Scholar]
- Reimer K. A., Lowe J. E., Rasmussen M. M., Jennings R. B. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977 Nov;56(5):786–794. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Fujiwara H., Onodera T., Wu D. J., Hamashima Y., Kawai C. Quantitative analysis of myocardial fibrosis in normals, hypertensive hearts, and hypertrophic cardiomyopathy. Br Heart J. 1986 Jun;55(6):575–581. doi: 10.1136/hrt.55.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Fujiwara H., Onodera T., Wu D. J., Matsuda M., Hamashima Y., Kawai C. Quantitative analysis of narrowings of intramyocardial small arteries in normal hearts, hypertensive hearts, and hearts with hypertrophic cardiomyopathy. Circulation. 1987 Jun;75(6):1130–1139. doi: 10.1161/01.cir.75.6.1130. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Fujiwara H., Fujiwara T., Ikehara S., Hamashima Y. Quantitative analysis of myocardial infarction in (NZW x BXSB)F1 hybrid mice with systemic lupus erythematosus and small coronary artery disease. Am J Pathol. 1987 Dec;129(3):477–485. [PMC free article] [PubMed] [Google Scholar]