Abstract
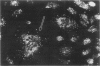
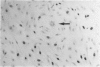
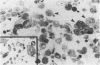
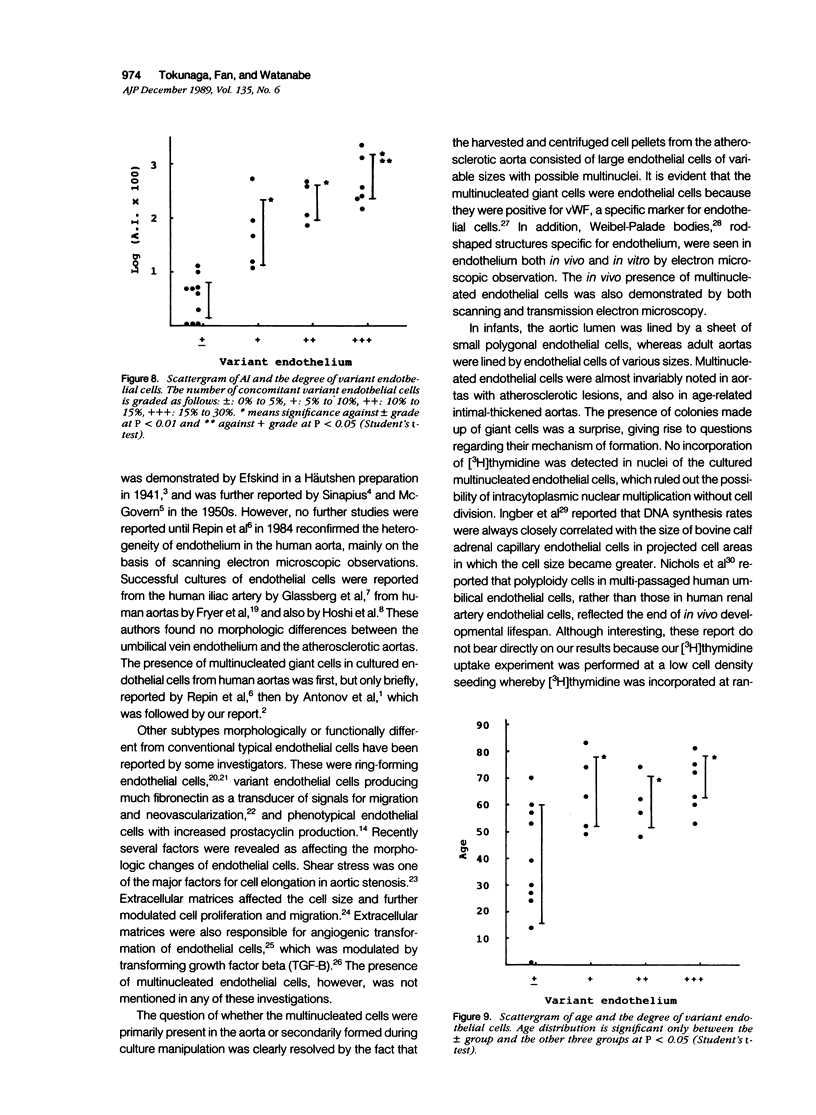
Endothelial cells were cultured from human aortas and inferior venae cavae of autopsied subjects ranging in age from infancy to 85 years. Endothelial cells in 32 of more than 100 attempted cultures were pure enough for evaluation. Emerged endothelial cells in primary culture were classified into two types: typical endothelium and variant endothelium. Typical endothelial cells were small, round to polygonal shaped, and were arranged uniformly. Their diameter ranged from 50 to 70 microns. Variant endothelial cells were larger, ranging from 100 to 200 microns in diameter, and giant endothelial cells measuring more than 250 microns in diameter were scattered among them. Variant endothelial cells were usually multinucleated and possessed endothelium-specific markers of vWF and Weibel-Palade bodies. No incorporation of [3H]thymidine was found in the nuclei of cultured variant endothelial cells. Although most cultured endothelial cells were of the typical type, variant endothelial cells were interspersed throughout the culture. The ratio of variant endothelial cells to typical cells correlated well with the severity of atherosclerosis, but less so with aging. The number of variant endothelial cells in cultures from inferior venae cavae was slight and constant throughout all age groups. The presence of multinucleated endothelial cells in in vivo aortas was confirmed by both scanning and transmission electron microscopy. They sometimes existed in colonies in the aortas from elderly subjects with intimal-thickened or advanced atherosclerotic lesions. These results indicate that variant endothelial cells were present in vivo and their ratio in primary culture reflected the in vivo population. It is likely that these cells were formed by adhesion of adjacent typical endothelial cells and that this process was affected more by atherosclerosis than by aging. Although it is not clear if the multinucleated variant cells were formed before the formation of atherosclerotic plaque or after the plaque formation, they will contribute to further development of atherosclerotic lesions, which in turn cause malfunction of the cell membrane. We suggest that there is a cyclic effect of these processes for multiplication of the variant endothelial cells and advancement of atherosclerotic lesions.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davison P. M., Bensch K., Karasek M. A. Isolation and growth of endothelial cells from the microvessels of the newborn human foreskin in cell culture. J Invest Dermatol. 1980 Oct;75(4):316–321. doi: 10.1111/1523-1747.ep12530941. [DOI] [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. C., Zetter B. R. Long-term culture of capillary endothelial cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5217–5221. doi: 10.1073/pnas.76.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. Angiogenesis in vitro. Nature. 1980 Dec 11;288(5791):551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- Fryer D. G., Birnbaum G., Luttrell C. N. Human endothelium in cell culture. J Atheroscler Res. 1966 Mar-Apr;6(2):151–163. doi: 10.1016/s0368-1319(66)80019-4. [DOI] [PubMed] [Google Scholar]
- GORE I., TEJADA C. The quantitative appraisal of atherosclerosis. Am J Pathol. 1957 Sep-Oct;33(5):875–885. [PMC free article] [PubMed] [Google Scholar]
- Glassberg M. K., Bern M. M., Coughlin S. R., Haudenschild C. C., Hoyer L. W., Antoniades H. N., Zetter B. R. Cultured endothelial cells derived from the human iliac arteries. In Vitro. 1982 Oct;18(10):859–866. doi: 10.1007/BF02796327. [DOI] [PubMed] [Google Scholar]
- Hahn G. L., Polgar P. R. Prostaglandin production in phenotypically distinct cultured bovine pulmonary artery endothelium. Atherosclerosis. 1984 Apr;51(1):143–150. doi: 10.1016/0021-9150(84)90150-3. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Madri J. A., Folkman J. Endothelial growth factors and extracellular matrix regulate DNA synthesis through modulation of cell and nuclear expansion. In Vitro Cell Dev Biol. 1987 May;23(5):387–394. doi: 10.1007/BF02620997. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A. Endothelial cells and the biology of factor VIII. N Engl J Med. 1977 Feb 17;296(7):377–383. doi: 10.1056/NEJM197702172960707. [DOI] [PubMed] [Google Scholar]
- Kubota Y., Kleinman H. K., Martin G. R., Lawley T. J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988 Oct;107(4):1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R. I., Jaffe E. A., Weksler B. B., Tack-Goldman K. Nitroglycerin stimulates synthesis of prostacyclin by cultured human endothelial cells. J Clin Invest. 1981 Mar;67(3):762–769. doi: 10.1172/JCI110093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARUYAMA Y. The human endothelial cell in tissue culture. Z Zellforsch Mikrosk Anat. 1963;60:69–79. doi: 10.1007/BF00329383. [DOI] [PubMed] [Google Scholar]
- Maciag T., Cerundolo J., Ilsley S., Kelley P. R., Forand R. An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5674–5678. doi: 10.1073/pnas.76.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M., Tucker A. M. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J Cell Biol. 1988 Apr;106(4):1375–1384. doi: 10.1083/jcb.106.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M., Yannariello-Brown J. Matrix-driven cell size change modulates aortic endothelial cell proliferation and sheet migration. Am J Pathol. 1988 Jul;132(1):18–27. [PMC free article] [PubMed] [Google Scholar]
- McAuslan B. R., Hannan G. N., Reilly W., Stewart F. H. Variant endothelial cells. Fibronectin as a transducer of signals for migration and neovascularisation. J Cell Physiol. 1980 Aug;104(2):177–186. doi: 10.1002/jcp.1041040207. [DOI] [PubMed] [Google Scholar]
- McGOVERN V. J. Reactions to injury of vascular endothelium with special reference to the problem of thrombosis. J Pathol Bacteriol. 1955 Jan-Apr;69(1-2):283–293. doi: 10.1002/path.1700690136. [DOI] [PubMed] [Google Scholar]
- Nichols W. W., Buynak E. B., Bradt C., Hill R., Aronson M., Jarrell B. E., Mueller S. N., Levine E. M. Cytogenetic evaluation of human endothelial cell cultures. J Cell Physiol. 1987 Sep;132(3):453–462. doi: 10.1002/jcp.1041320307. [DOI] [PubMed] [Google Scholar]
- Repin V. S., Dolgov V. V., Zaikina O. E., Novikov I. D., Antonov A. S., Nikolaeva M. A., Smirnov V. N. Heterogeneity of endothelium in human aorta. A quantitative analysis by scanning electron microscopy. Atherosclerosis. 1984 Jan;50(1):35–52. doi: 10.1016/0021-9150(84)90006-6. [DOI] [PubMed] [Google Scholar]
- SINAPIUS D. Uber das Aortenendothel. Virchows Arch Pathol Anat Physiol Klin Med. 1952;322(6):662–694. doi: 10.1007/BF00957503. [DOI] [PubMed] [Google Scholar]
- Taylor C. R., Russell R., Chandor S. An immunohistologic study of multiple myeloma and related conditions, using an immunoperoxidase method. Am J Clin Pathol. 1978 Oct;70(4):612–622. doi: 10.1093/ajcp/70.4.612. [DOI] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Tokunaga O., Fan J. L., Watanabe T. Atherosclerosis and endothelium. Part II. Properties of aortic endothelial and smooth muscle cells cultured at various ambient pressures. Acta Pathol Jpn. 1989 Jun;39(6):356–362. doi: 10.1111/j.1440-1827.1989.tb02447.x. [DOI] [PubMed] [Google Scholar]
- Tokunaga O., Morimatsu M., Nakashima T. Ring formation by human variant endothelial cells in vitro. Br J Exp Pathol. 1984 Apr;65(2):165–170. [PMC free article] [PubMed] [Google Scholar]
- Tokunaga O., Watanabe T. Atherosclerosis and endothelium. Part 1. A simple method of endothelial cell culture from human atherosclerotic aorta. Acta Pathol Jpn. 1987 Apr;37(4):527–536. [PubMed] [Google Scholar]
- WEIBEL E. R., PALADE G. E. NEW CYTOPLASMIC COMPONENTS IN ARTERIAL ENDOTHELIA. J Cell Biol. 1964 Oct;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zand T., Nunnari J. J., Hoffman A. H., Savilonis B. J., MacWilliams B., Majno G., Joris I. Endothelial adaptations in aortic stenosis. Correlation with flow parameters. Am J Pathol. 1988 Nov;133(2):407–418. [PMC free article] [PubMed] [Google Scholar]