Abstract
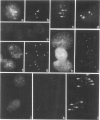
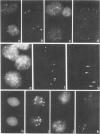
The nuclear DNA content of 53 transitional cell carcinomas (TCCs) of the urinary bladder, as determined by flow cytometry (FCM), was compared with chromosome ploidy as detected by nonradioactive in situ hybridization (ISH). For this purpose, probes for repetitive DNA targets in the (peri) centromeric region of chromosomes 1 and 18 were used. Hybridization results with both probes of 35 TCCs, which had a DNA index of approximately 1.0 as concluded from FCM, showed evident chromosome 1 aberrations in approximately 25% of the tumors, and in a few cases an aberration for chromosome 18 was detected. Comparison of the ISH spot numbers for both chromosomes showed in most cases a higher number for chromosome 1 than for chromosome 18. ISH on 18 cases of TCCs, which showed a single peak in FCM with a DNA-index of 1.2 to 3.2, exhibited a profound heterogeneity. In these TCCs the ratio between chromosomes 1 and 18 varied over a wide range, resulting in cases showing more hybridization signals for chromosome 1 than for chromosome 18 or the opposite. Furthermore, using ISH minor cell populations showing polyploidization and giant cells containing numerous ISH signals could occasionally be detected. Results showed that interphase cytogenetics by ISH enable a fast screening of numerical chromosome aberrations and detection of different cell populations within one tumor, which was apparently homogeneous according to FCM.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin N. B., Baker M. C. Cytogenetic study of ten carcinomas of the bladder: involvement of chromosomes 1 and 11. Cancer Genet Cytogenet. 1985 Feb 15;15(3-4):253–268. doi: 10.1016/0165-4608(85)90169-4. [DOI] [PubMed] [Google Scholar]
- Atkin N. B. Chromosome 1 aberrations in cancer. Cancer Genet Cytogenet. 1986 Apr 15;21(4):279–285. doi: 10.1016/0165-4608(86)90206-2. [DOI] [PubMed] [Google Scholar]
- Babu V. R., Lutz M. D., Miles B. J., Farah R. N., Weiss L., Van Dyke D. L. Tumor behavior in transitional cell carcinoma of the bladder in relation to chromosomal markers and histopathology. Cancer Res. 1987 Dec 15;47(24 Pt 1):6800–6805. [PubMed] [Google Scholar]
- Barlogie B., Raber M. N., Schumann J., Johnson T. S., Drewinko B., Swartzendruber D. E., Göhde W., Andreeff M., Freireich E. J. Flow cytometry in clinical cancer research. Cancer Res. 1983 Sep;43(9):3982–3997. [PubMed] [Google Scholar]
- Brigati D. J., Myerson D., Leary J. J., Spalholz B., Travis S. Z., Fong C. K., Hsiung G. D., Ward D. C. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotin-labeled hybridization probes. Virology. 1983 Apr 15;126(1):32–50. doi: 10.1016/0042-6822(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Bubeník J., Baresová M., Viklický V., Jakoubková J., Sainerová H., Donner J. Established cell line of urinary bladder carcinoma (T24) containing tumour-specific antigen. Int J Cancer. 1973 May;11(3):765–773. doi: 10.1002/ijc.2910110327. [DOI] [PubMed] [Google Scholar]
- Burns J., Redfern D. R., Esiri M. M., McGee J. O. Human and viral gene detection in routine paraffin embedded tissue by in situ hybridisation with biotinylated probes: viral localisation in herpes encephalitis. J Clin Pathol. 1986 Oct;39(10):1066–1073. doi: 10.1136/jcp.39.10.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke H. J., Hindley J. Cloning of human satellite III DNA: different components are on different chromosomes. Nucleic Acids Res. 1979 Jul 25;6(10):3177–3197. doi: 10.1093/nar/6.10.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R., Irving C. C. Inhibition of DNA methylation by S-adenosylethionine with the production of methyl-deficient DNA in regenerating rat liver. Cancer Res. 1977 Jan;37(1):222–225. [PubMed] [Google Scholar]
- Cremer T., Landegent J., Brückner A., Scholl H. P., Schardin M., Hager H. D., Devilee P., Pearson P., van der Ploeg M. Detection of chromosome aberrations in the human interphase nucleus by visualization of specific target DNAs with radioactive and non-radioactive in situ hybridization techniques: diagnosis of trisomy 18 with probe L1.84. Hum Genet. 1986 Dec;74(4):346–352. doi: 10.1007/BF00280484. [DOI] [PubMed] [Google Scholar]
- Cremer T., Lichter P., Borden J., Ward D. C., Manuelidis L. Detection of chromosome aberrations in metaphase and interphase tumor cells by in situ hybridization using chromosome-specific library probes. Hum Genet. 1988 Nov;80(3):235–246. doi: 10.1007/BF01790091. [DOI] [PubMed] [Google Scholar]
- Cremer T., Tesin D., Hopman A. H., Manuelidis L. Rapid interphase and metaphase assessment of specific chromosomal changes in neuroectodermal tumor cells by in situ hybridization with chemically modified DNA probes. Exp Cell Res. 1988 Jun;176(2):199–220. doi: 10.1016/0014-4827(88)90325-4. [DOI] [PubMed] [Google Scholar]
- Devilee P., Cremer T., Slagboom P., Bakker E., Scholl H. P., Hager H. D., Stevenson A. F., Cornelisse C. J., Pearson P. L. Two subsets of human alphoid repetitive DNA show distinct preferential localization in the pericentric regions of chromosomes 13, 18, and 21. Cytogenet Cell Genet. 1986;41(4):193–201. doi: 10.1159/000132229. [DOI] [PubMed] [Google Scholar]
- Devilee P., Thierry R. F., Kievits T., Kolluri R., Hopman A. H., Willard H. F., Pearson P. L., Cornelisse C. J. Detection of chromosome aneuploidy in interphase nuclei from human primary breast tumors using chromosome-specific repetitive DNA probes. Cancer Res. 1988 Oct 15;48(20):5825–5830. [PubMed] [Google Scholar]
- Fossa S. D., Kaalhus O. Nuclear size and chromatin concentration in transitional cell carcinoma of the human urinary bladder. Beitr Pathol. 1976 Apr;157(2):109–125. doi: 10.1016/s0005-8165(76)80098-4. [DOI] [PubMed] [Google Scholar]
- Hopman A. H., Ramaekers F. C., Raap A. K., Beck J. L., Devilee P., van der Ploeg M., Vooijs G. P. In situ hybridization as a tool to study numerical chromosome aberrations in solid bladder tumors. Histochemistry. 1988;89(4):307–316. doi: 10.1007/BF00500631. [DOI] [PubMed] [Google Scholar]
- Hopman A. H., Wiegant J., Raap A. K., Landegent J. E., van der Ploeg M., van Duijn P. Bi-color detection of two target DNAs by non-radioactive in situ hybridization. Histochemistry. 1986;85(1):1–4. doi: 10.1007/BF00508646. [DOI] [PubMed] [Google Scholar]
- Hopman A. H., Wiegant J., Tesser G. I., Van Duijn P. A non-radioactive in situ hybridization method based on mercurated nucleic acid probes and sulfhydryl-hapten ligands. Nucleic Acids Res. 1986 Aug 26;14(16):6471–6488. doi: 10.1093/nar/14.16.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopman A. H., Wiegant J., van Duijn P. Mercurated nucleic acid probes, a new principle for non-radioactive in situ hybridization. Exp Cell Res. 1987 Apr;169(2):357–368. doi: 10.1016/0014-4827(87)90196-0. [DOI] [PubMed] [Google Scholar]
- Kovacs G. Serial cytogenetic analysis in a patient with pseudodiploid bladder cancer. J Cancer Res Clin Oncol. 1985;110(3):249–251. doi: 10.1007/BF00399284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel D. E., Dressler L. G., McGuire W. L. Flow cytometry, cellular DNA content, and prognosis in human malignancy. J Clin Oncol. 1987 Oct;5(10):1690–1703. doi: 10.1200/JCO.1987.5.10.1690. [DOI] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Smeets A. W., Pauwels R. P., Beck H. L., Feitz W. F., Geraedts J. P., Debruyne F. M., Laarakkers L., Vooijs G. P., Ramaekers F. C. Comparison of tissue disaggregation techniques of transitional cell bladder carcinomas for flow cytometry and chromosomal analysis. Cytometry. 1987 Jan;8(1):14–19. doi: 10.1002/cyto.990080103. [DOI] [PubMed] [Google Scholar]
- Smeets A. W., Pauwels R. P., Beck J. L., Geraedts J. P., Debruyne F. M., Laarakkers L., Feitz W. F., Vooijs G. P., Ramaekers F. C. Tissue-specific markers in flow cytometry of urological cancers. III. Comparing chromosomal and flow cytometric DNA analysis of bladder tumors. Int J Cancer. 1987 Mar 15;39(3):304–310. doi: 10.1002/ijc.2910390307. [DOI] [PubMed] [Google Scholar]
- Smeets W., Pauwels R., Laarakkers L., Debruyne F., Geraedts J. Chromosomal analysis of bladder cancer. III. Nonrandom alterations. Cancer Genet Cytogenet. 1987 Nov;29(1):29–41. doi: 10.1016/0165-4608(87)90028-8. [DOI] [PubMed] [Google Scholar]
- Summers J. L., Falor W. H., Ward R. A 10-year analysis of chromosomes in non-invasive papillary carcinoma of the bladder. J Urol. 1981 Feb;125(2):177–178. doi: 10.1016/s0022-5347(17)54954-x. [DOI] [PubMed] [Google Scholar]
- Teyssier J. R. The chromosomal analysis of human solid tumors. A triple challenge. Cancer Genet Cytogenet. 1989 Jan;37(1):103–125. doi: 10.1016/0165-4608(89)90080-0. [DOI] [PubMed] [Google Scholar]
- Tribukait B., Granberg-Ohman I., Wijkström H. Flow cytometric DNA and cytogenetic studies in human tumors: a comparison and discussion of the differences in modal values obtained by the two methods. Cytometry. 1986 Mar;7(2):194–199. doi: 10.1002/cyto.990070211. [DOI] [PubMed] [Google Scholar]
- Vanni R., Scarpa R. M., Nieddu M., Usai E. Cytogenetic investigation on 30 bladder carcinomas. Cancer Genet Cytogenet. 1988 Jan;30(1):35–42. doi: 10.1016/0165-4608(88)90090-8. [DOI] [PubMed] [Google Scholar]
- Wijkström H., Granberg-Ohman I., Tribukait B. Chromosomal and DNA patterns in transitional cell bladder carcinoma. A comparative cytogenetic and flow-cytofluorometric DNA study. Cancer. 1984 Apr 15;53(8):1718–1723. doi: 10.1002/1097-0142(19840415)53:8<1718::aid-cncr2820530817>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]