Abstract
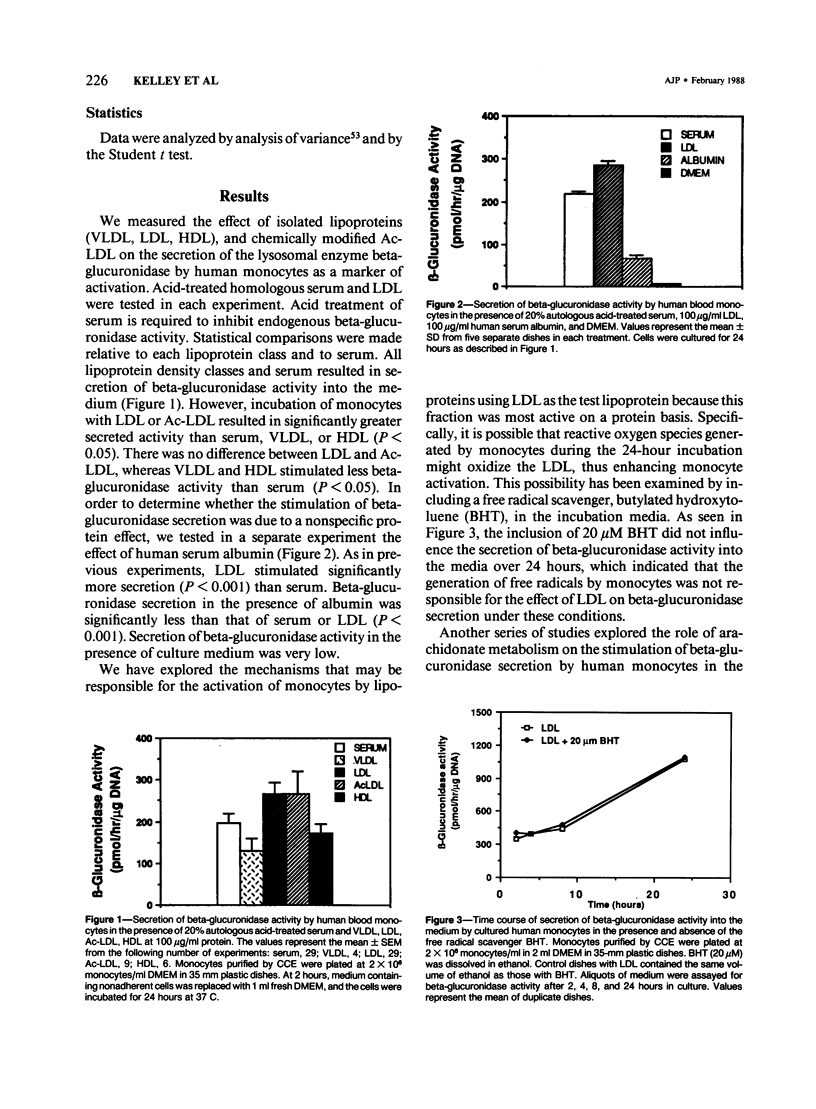
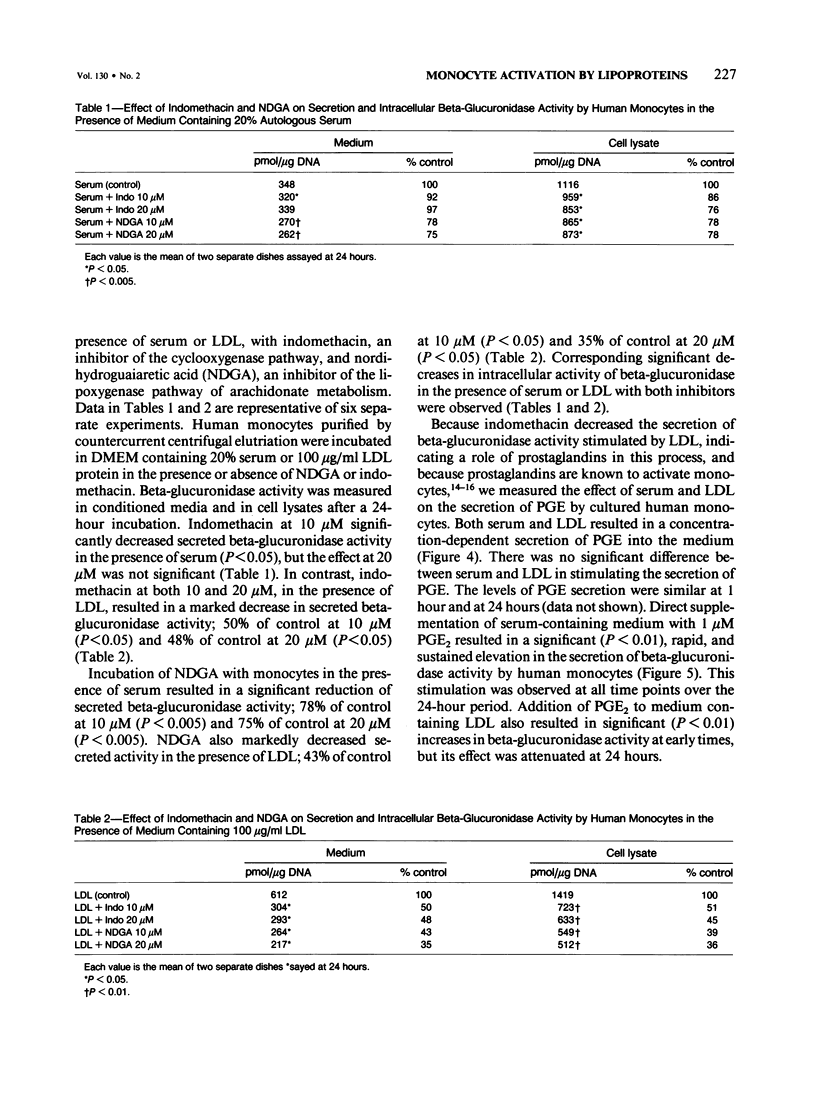
Activation of human peripheral blood monocytes could enhance their attachment and or migration into the arterial intima and their various secretory and other functions, thus influencing the pathogenesis of atherosclerosis. In these experiments the authors have explored the role of lipoproteins in the activation of human blood monocytes. Monocytes were purified from citrated blood by Histopaque density gradient centrifugation and countercurrent centrifugal elutriation and cultured in DMEM in the presence of 20% acid-treated autologous serum or 100 micrograms/ml each of VLDL, LDL, Ac-LDL, and HDL. Secretion of beta-glucuronidase activity into the media was measured as a marker of activation. All of the lipoprotein density classes as well as serum stimulated secretion of beta-glucuronidase activity, with LDL and Ac-LDL having a greater influence than serum, VLDL, or HDL. Serum and LDL also stimulated secretion of prostaglandin E into the culture medium. Incubation of monocytes with serum or LDL in the presence of inhibitors of arachidonate metabolism (NDGA and indomethacin) resulted in a significant decrease in secreted and intracellular beta-glucuronidase activity, indicating a role for products of arachidonate metabolism in the activation of monocytes by lipoproteins.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berton G., Gordon S. Superoxide release by peritoneal and bone marrow-derived mouse macrophages. Modulation by adherence and cell activation. Immunology. 1983 Aug;49(4):693–704. [PMC free article] [PubMed] [Google Scholar]
- Bonney R. J., Wightman P. D., Davies P., Sadowski S. J., Kuehl F. A., Jr, Humes J. L. Regulation of prostaglandin synthesis and of the selective release of lysosomal hydrolases by mouse peritoneal macrophages. Biochem J. 1978 Nov 15;176(2):433–442. doi: 10.1042/bj1760433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Basu S. K., Falck J. R., Ho Y. K., Goldstein J. L. The scavenger cell pathway for lipoprotein degradation: specificity of the binding site that mediates the uptake of negatively-charged LDL by macrophages. J Supramol Struct. 1980;13(1):67–81. doi: 10.1002/jss.400130107. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L., Krieger M., Ho Y. K., Anderson R. G. Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J Cell Biol. 1979 Sep;82(3):597–613. doi: 10.1083/jcb.82.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camussi G., Bussolino F., Tetta C., Piacibello W., Aglietta M. Biosynthesis and release of platelet-activating factor from human monocytes. Int Arch Allergy Appl Immunol. 1983 Mar;70(3):245–251. doi: 10.1159/000233331. [DOI] [PubMed] [Google Scholar]
- Chait A., Henze K., Mazzone T., Jensen M., Hammond W. Low density lipoprotein receptor activity in freshly isolated human blood monocytes and lymphocytes. Metabolism. 1982 Jul;31(7):721–727. doi: 10.1016/0026-0495(82)90204-9. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A. The macrophage--versatile element of inflammation. Harvey Lect. 1981 1982;77:63–80. [PubMed] [Google Scholar]
- Coleman D. L. Regulation of macrophage phagocytosis. Eur J Clin Microbiol. 1986 Feb;5(1):1–5. doi: 10.1007/BF02013451. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Stephenson M. L., Schmidt E., Karge W., Krane S. M. Purification of a factor from human blood monocyte-macrophages which stimulates the production of collagenase and prostaglandin E2 by cells cultured from rheumatoid synovial tissues. FEBS Lett. 1981 Feb 23;124(2):253–253. doi: 10.1016/0014-5793(81)80149-4. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Chang D., Griffin G. L., Heinrikson R. L., Kaiser E. T. Platelet factor 4 is chemotactic for neutrophils and monocytes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4584–4587. doi: 10.1073/pnas.78.7.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier J. C., Tenu J. P., Lemaire G., Petit J. F. Regulation of plasminogen activator secretion in mouse peritoneal macrophages. I. - Role of serum studied by a new spectrophotometric assay for plasminogen activators. Biochimie. 1979;61(4):463–471. doi: 10.1016/s0300-9084(79)80202-3. [DOI] [PubMed] [Google Scholar]
- Ferreri N. R., Howland W. C., Spiegelberg H. L. Release of leukotrienes C4 and B4 and prostaglandin E2 from human monocytes stimulated with aggregated IgG, IgA, and IgE. J Immunol. 1986 Jun 1;136(11):4188–4193. [PubMed] [Google Scholar]
- Fogelman A. M., Haberland M. E., Seager J., Hokom M., Edwards P. A. Factors regulating the activities of the low density lipoprotein receptor and the scavenger receptor on human monocyte-macrophages. J Lipid Res. 1981 Sep;22(7):1131–1141. [PubMed] [Google Scholar]
- Fogelman A. M., Seager J., Groopman J. E., Berliner J. A., Haberland M. E., Edwards P. A., Golde D. W. Lymphokines secreted by an established lymphocyte line modulate receptor-mediated endocytosis in macrophages derived from human monocytes. J Immunol. 1983 Nov;131(5):2368–2373. [PubMed] [Google Scholar]
- Fogelman A. M., Seager J., Hokom M., Edwards P. A. Separation of and cholesterol synthesis by human lymphocytes and monocytes. J Lipid Res. 1979 Mar;20(3):379–388. [PubMed] [Google Scholar]
- Gordon S. Biology of the macrophage. J Cell Sci Suppl. 1986;4:267–286. doi: 10.1242/jcs.1986.supplement_4.16. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung H. P., Kladetzky R. G., Hennerici M. Chemically modified low density lipoproteins as inducers of enzyme release from macrophages. FEBS Lett. 1985 Jul 8;186(2):211–214. doi: 10.1016/0014-5793(85)80710-9. [DOI] [PubMed] [Google Scholar]
- Hartung H. P., Kladetzky R. G., Melnik B., Hennerici M. Stimulation of the scavenger receptor on monocytes-macrophages evokes release of arachidonic acid metabolites and reduced oxygen species. Lab Invest. 1986 Aug;55(2):209–216. [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6499–6503. doi: 10.1073/pnas.78.10.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler J. R., Morel D. W., Lewis L. J., Chisolm G. M. Lipoprotein oxidation and lipoprotein-induced cytotoxicity. Arteriosclerosis. 1983 May-Jun;3(3):215–222. doi: 10.1161/01.atv.3.3.215. [DOI] [PubMed] [Google Scholar]
- Hoover R. L., Folger R., Haering W. A., Ware B. R., Karnovsky M. J. Adhesion of leukocytes to endothelium: roles of divalent cations, surface charge, chemotactic agents and substrate. J Cell Sci. 1980 Oct;45:73–86. doi: 10.1242/jcs.45.1.73. [DOI] [PubMed] [Google Scholar]
- Huber H., Fudenberg H. H. Receptor sites of human monocytes for IgG. Int Arch Allergy Appl Immunol. 1968;34(1):18–31. doi: 10.1159/000230091. [DOI] [PubMed] [Google Scholar]
- Janco R. L., Morris P. J. Regulation of monocyte procoagulant by chemoattractants. Blood. 1985 Mar;65(3):545–552. [PubMed] [Google Scholar]
- Johnson W. J., Marino P. A., Schreiber R. D., Adams D. O. Sequential activation of murine mononuclear phagocytes for tumor cytolysis: differential expression of markers by macrophages in the several stages of development. J Immunol. 1983 Aug;131(2):1038–1043. [PubMed] [Google Scholar]
- Kaplan G., Gaudernack G. In vitro differentiation of human monocytes. Differences in monocyte phenotypes induced by cultivation on glass or on collagen. J Exp Med. 1982 Oct 1;156(4):1101–1114. doi: 10.1084/jem.156.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. E., Saba T. M. Macrophage metabolism of fibrin: role of fibronectin. Surv Immunol Res. 1984;3(2-3):165–168. doi: 10.1007/BF02918785. [DOI] [PubMed] [Google Scholar]
- Kelley J. L., Alaupovic P. Lipid transport in the avian species. Part I. Isolation and characterization of apolipoproteins and major lipoprotein density classes of male turkey serum. Atherosclerosis. 1976 Jul-Aug;24(1-2):155–175. doi: 10.1016/0021-9150(76)90073-3. [DOI] [PubMed] [Google Scholar]
- Kelley J. L., Rozek M. M., Suenram C. A., Schwartz C. J. Activation of human blood monocytes by adherence to tissue culture plastic surfaces. Exp Mol Pathol. 1987 Jun;46(3):266–278. doi: 10.1016/0014-4800(87)90049-9. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med. 1978 Mar 1;147(3):952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lasser A. The mononuclear phagocytic system: a review. Hum Pathol. 1983 Feb;14(2):108–126. doi: 10.1016/s0046-8177(83)80239-1. [DOI] [PubMed] [Google Scholar]
- Leslie C. C., Detty D. M. Arachidonic acid turnover in response to lipopolysaccharide and opsonized zymosan in human monocyte-derived macrophages. Biochem J. 1986 May 15;236(1):251–259. doi: 10.1042/bj2360251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Brown M. S., Ho Y. K., Goldstein J. L. Cholesteryl ester synthesis in macrophages: stimulation by beta-very low density lipoproteins from cholesterol-fed animals of several species. J Lipid Res. 1980 Nov;21(8):970–980. [PubMed] [Google Scholar]
- Mazzone T., Jensen M., Chait A. Human arterial wall cells secrete factors that are chemotactic for monocytes. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5094–5097. doi: 10.1073/pnas.80.16.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M. K., Meats J. E., Ebsworth N. M., Gowen M., Murphy G., Reynolds J. J., Russell R. G. Interactions in connective tissues involving monocyte/macrophages and control of production of proteinases and proteinase inhibitors. Agents Actions Suppl. 1982;11:131–138. [PubMed] [Google Scholar]
- Morel D. W., DiCorleto P. E., Chisolm G. M. Endothelial and smooth muscle cells alter low density lipoprotein in vitro by free radical oxidation. Arteriosclerosis. 1984 Jul-Aug;4(4):357–364. doi: 10.1161/01.atv.4.4.357. [DOI] [PubMed] [Google Scholar]
- Morel D. W., Hessler J. R., Chisolm G. M. Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. J Lipid Res. 1983 Aug;24(8):1070–1076. [PubMed] [Google Scholar]
- Musson R. A. Human serum induces maturation of human monocytes in vitro. Changes in cytolytic activity, intracellular lysosomal enzymes, and nonspecific esterase activity. Am J Pathol. 1983 Jun;111(3):331–340. [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., DeSantis N. M., Nogueira N., Nathan C. F. Lymphokines enhance the capacity of human monocytes to secret reactive oxygen intermediates. J Clin Invest. 1982 Nov;70(5):1042–1048. doi: 10.1172/JCI110691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan J. C., Pickett W. C. Studies on the effects of cyclo-oxygenase and lipoxygenase inhibitors on the macrophage stimulated synthesis of collagenase by rabbit chondrocytes. Agents Actions. 1985 Oct;17(1):73–76. doi: 10.1007/BF01966685. [DOI] [PubMed] [Google Scholar]
- Orlandi M., Bartolini G., Chiricolo M., Minghetti L., Franceschi C., Tomasi V. Prostaglandin and thromboxane biosynthesis in isolated platelet-free human monocytes. I. A modified procedure for the characterization of the prostaglandin spectrum produced by resting and activated monocytes. Prostaglandins Leukot Med. 1985 May;18(2):205–216. doi: 10.1016/0262-1746(85)90020-4. [DOI] [PubMed] [Google Scholar]
- Pace J. L., Taffet S. M., Russell S. W. The effect of endotoxin in eliciting agents on the activation of mouse macrophages for tumor cell killing. J Reticuloendothel Soc. 1981 Jul;30(1):15–21. [PubMed] [Google Scholar]
- Passwell J. H., Dayer J. M., Merler E. Increased prostaglandin production by human monocytes after membrane receptor activation. J Immunol. 1979 Jul;123(1):115–120. [PubMed] [Google Scholar]
- Raymond T. L., Reynolds S. A., Swanson J. A. Lipoproteins of the extravascular space: enhanced macrophage degradation of low density lipoproteins from interstitial inflammatory fluid. J Lipid Res. 1985 Nov;26(11):1356–1362. [PubMed] [Google Scholar]
- Ridge S. C., Oronsky A. L., Kerwar S. S. Induction of the synthesis of latent collagenase and latent neutral protease in chondrocytes by a factor synthesized by activated macrophages. Arthritis Rheum. 1980 Apr;23(4):448–454. doi: 10.1002/art.1780230407. [DOI] [PubMed] [Google Scholar]
- Sanderson R. J., Shepperdson R. T., Vatter A. E., Talmage D. W. Isolation and enumeration of peripheral blood monocytes. J Immunol. 1977 Apr;118(4):1409–1414. [PubMed] [Google Scholar]
- Schnyder J., Baggiolini M. Secretion of lysosomal hydrolases by stimulated and nonstimulated macrophages. J Exp Med. 1978 Aug 1;148(2):435–450. doi: 10.1084/jem.148.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorlemmer H. U., Burger R., Hylton W., Allison A. C. Induction of lysosomal enzyme release from cultured macrophages by dextran sulfate. Clin Immunol Immunopathol. 1977 Jan;7(1):88–96. doi: 10.1016/0090-1229(77)90033-2. [DOI] [PubMed] [Google Scholar]
- Schorlemmer H. U., Davies P., Hylton W., Gugig M., Allison A. C. The selective release of lysosomal acid hydrolases from mouse peritoneal macrophages by stimuli of chronic inflammation. Br J Exp Pathol. 1977 Jun;58(3):315–326. [PMC free article] [PubMed] [Google Scholar]
- Snider M. E., Fertel R. H., Zwilling B. S. Prostaglandin regulation of macrophage function: effect of endogenous and exogenous prostaglandins. Cell Immunol. 1982 Dec;74(2):234–242. doi: 10.1016/0008-8749(82)90024-7. [DOI] [PubMed] [Google Scholar]
- Sorger T., Germinario R. J. A direct solubilization procedure for the determination of DNA and protein in cultured fibroblast monolayers. Anal Biochem. 1983 May;131(1):254–256. doi: 10.1016/0003-2697(83)90163-x. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura R., Werb Z. Secretory products of macrophages and their physiological functions. Am J Physiol. 1984 Jan;246(1 Pt 1):C1–C9. doi: 10.1152/ajpcell.1984.246.1.C1. [DOI] [PubMed] [Google Scholar]
- Van Lenten B. J., Fogelman A. M., Seager J., Ribi E., Haberland M. E., Edwards P. A. Bacterial endotoxin selectively prevents the expression of scavenger-receptor activity on human monocyte-macrophages. J Immunol. 1985 Jun;134(6):3718–3721. [PubMed] [Google Scholar]
- Werb Z., Chin J. R. Endotoxin suppresses expression of apoprotein E by mouse macrophages in vivo and in culture. A biochemical and genetic study. J Biol Chem. 1983 Sep 10;258(17):10642–10648. [PubMed] [Google Scholar]
- Werb Z., Gordon S. Secretion of a specific collagenase by stimulated macrophages. J Exp Med. 1975 Aug 1;142(2):346–360. doi: 10.1084/jem.142.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollen J. W., Walker P. G. The fluorimetric estimation of beta-glucuronidase in blood plasma. Clin Chim Acta. 1965 Dec;12(6):659–670. doi: 10.1016/0009-8981(65)90148-8. [DOI] [PubMed] [Google Scholar]
- van Ginkel C. J., van Aken W. G., Oh J. I., Vreeken J. Stimulation of monocyte procoagulant activity by adherence to different surfaces. Br J Haematol. 1977 Sep;37(1):35–45. [PubMed] [Google Scholar]


