Abstract
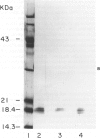
The basement membranes of bovine cornea are found to contain an angiogenic endothelial cell mitogen, basic fibroblast growth factor (FGF), as determined by heparin-affinity chromatography, immunoblotting, immunofluorescence, and stimulation of capillary endothelial cell proliferation. The growth factor appears to be bound to heparan sulfate and is released from the cornea by treatment with heparin, a hexasaccharide heparin fragment, heparan sulfate, or heparanase, but not by chondroitin sulfate or chondroitinase. These findings indicate that basement membranes of the cornea may serve as physiologic storage depots for an angiogenic molecule. Abnormal release of this growth factor could be responsible for corneal neovascularization in a variety of ocular diseases. Physiologic and pathologic neovascularization in other tissues may also be initiated by release of stored angiogenic factors from the basement membrane. The sequestration of angiogenic endothelial mitogens in the basement membrane may be a general mechanism for regulating their accessibility to vascular endothelium.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azizkhan R. G., Azizkhan J. C., Zetter B. R., Folkman J. Mast cell heparin stimulates migration of capillary endothelial cells in vitro. J Exp Med. 1980 Oct 1;152(4):931–944. doi: 10.1084/jem.152.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird A., Esch F., Böhlen P., Ling N., Gospodarowicz D. Isolation and partial characterization of an endothelial cell growth factor from the bovine kidney: homology with basic fibroblast growth factor. Regul Pept. 1985 Nov 7;12(3):201–213. doi: 10.1016/0167-0115(85)90061-8. [DOI] [PubMed] [Google Scholar]
- Baird A., Mormède P., Böhlen P. Immunoreactive fibroblast growth factor in cells of peritoneal exudate suggests its identity with macrophage-derived growth factor. Biochem Biophys Res Commun. 1985 Jan 16;126(1):358–364. doi: 10.1016/0006-291x(85)90614-x. [DOI] [PubMed] [Google Scholar]
- Banda M. J., Knighton D. R., Hunt T. K., Werb Z. Isolation of a nonmitogenic angiogenesis factor from wound fluid. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7773–7777. doi: 10.1073/pnas.79.24.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courty J., Loret C., Moenner M., Chevallier B., Lagente O., Courtois Y., Barritault D. Bovine retina contains three growth factor activities with different affinity to heparin: eye derived growth factor I, II, III. Biochimie. 1985 Feb;67(2):265–269. doi: 10.1016/s0300-9084(85)80056-0. [DOI] [PubMed] [Google Scholar]
- D'Amore P. A., Klagsbrun M. Endothelial cell mitogens derived from retina and hypothalamus: biochemical and biological similarities. J Cell Biol. 1984 Oct;99(4 Pt 1):1545–1549. doi: 10.1083/jcb.99.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch F., Baird A., Ling N., Ueno N., Hill F., Denoroy L., Klepper R., Gospodarowicz D., Böhlen P., Guillemin R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6507–6511. doi: 10.1073/pnas.82.19.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. C., Zetter B. R. Long-term culture of capillary endothelial cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5217–5221. doi: 10.1073/pnas.76.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Langer R., Linhardt R. J., Haudenschild C., Taylor S. Angiogenesis inhibition and tumor regression caused by heparin or a heparin fragment in the presence of cortisone. Science. 1983 Aug 19;221(4612):719–725. doi: 10.1126/science.6192498. [DOI] [PubMed] [Google Scholar]
- Folkman J. What is the role of angiogenesis in metastasis from cutaneous melanoma? Eur J Cancer Clin Oncol. 1987 Apr;23(4):361–363. doi: 10.1016/0277-5379(87)90370-1. [DOI] [PubMed] [Google Scholar]
- Gimenez-Gallego G., Rodkey J., Bennett C., Rios-Candelore M., DiSalvo J., Thomas K. Brain-derived acidic fibroblast growth factor: complete amino acid sequence and homologies. Science. 1985 Dec 20;230(4732):1385–1388. doi: 10.1126/science.4071057. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol. 1986 Sep;128(3):475–484. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Gonzalez R., Fujii D. K. Are factors originating from serum, plasma, or cultured cells involved in the growth-promoting effect of the extracellular matrix produced by cultured bovine corneal endothelial cells? J Cell Physiol. 1983 Feb;114(2):191–202. doi: 10.1002/jcp.1041140208. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Madri J. A., Jamieson J. D. Role of basal lamina in neoplastic disorganization of tissue architecture. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3901–3905. doi: 10.1073/pnas.78.6.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M., Sasse J., Sullivan R., Smith J. A. Human tumor cells synthesize an endothelial cell growth factor that is structurally related to basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2448–2452. doi: 10.1073/pnas.83.8.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M., Shing Y. Heparin affinity of anionic and cationic capillary endothelial cell growth factors: analysis of hypothalamus-derived growth factors and fibroblast growth factors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):805–809. doi: 10.1073/pnas.82.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer P. M. Heparan sulfates of cultured cells. I. Membrane-associated and cell-sap species in Chinese hamster cells. Biochemistry. 1971 Apr 13;10(8):1437–1445. doi: 10.1021/bi00784a026. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Vogel K. G., Nicolson G. L. Solubilization and degradation of subendothelial matrix glycoproteins and proteoglycans by metastatic tumor cells. J Biol Chem. 1982 Mar 10;257(5):2678–2686. [PubMed] [Google Scholar]
- Lobb R. R., Fett J. W. Purification of two distinct growth factors from bovine neural tissue by heparin affinity chromatography. Biochemistry. 1984 Dec 18;23(26):6295–6299. doi: 10.1021/bi00321a001. [DOI] [PubMed] [Google Scholar]
- Maciag T., Mehlman T., Friesel R., Schreiber A. B. Heparin binds endothelial cell growth factor, the principal endothelial cell mitogen in bovine brain. Science. 1984 Aug 31;225(4665):932–935. doi: 10.1126/science.6382607. [DOI] [PubMed] [Google Scholar]
- Moscatelli D., Presta M., Rifkin D. B. Purification of a factor from human placenta that stimulates capillary endothelial cell protease production, DNA synthesis, and migration. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2091–2095. doi: 10.1073/pnas.83.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader H. B., Dietrich C. P., Buonassisi V., Colburn P. Heparin sequences in the heparan sulfate chains of an endothelial cell proteoglycan. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3565–3569. doi: 10.1073/pnas.84.11.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polverini P. J., Leibovich S. J. Induction of neovascularization in vivo and endothelial proliferation in vitro by tumor-associated macrophages. Lab Invest. 1984 Dec;51(6):635–642. [PubMed] [Google Scholar]
- Schreiber A. B., Kenney J., Kowalski W. J., Friesel R., Mehlman T., Maciag T. Interaction of endothelial cell growth factor with heparin: characterization by receptor and antibody recognition. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6138–6142. doi: 10.1073/pnas.82.18.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing Y., Folkman J., Haudenschild C., Lund D., Crum R., Klagsbrun M. Angiogenesis is stimulated by a tumor-derived endothelial cell growth factor. J Cell Biochem. 1985;29(4):275–287. doi: 10.1002/jcb.240290402. [DOI] [PubMed] [Google Scholar]
- Shing Y., Folkman J., Sullivan R., Butterfield C., Murray J., Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984 Mar 23;223(4642):1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- Srivastava A., Laidler P., Hughes L. E., Woodcock J., Shedden E. J. Neovascularization in human cutaneous melanoma: a quantitative morphological and Doppler ultrasound study. Eur J Cancer Clin Oncol. 1986 Oct;22(10):1205–1209. doi: 10.1016/0277-5379(86)90322-6. [DOI] [PubMed] [Google Scholar]
- Sullivan R., Klagsbrun M. Purification of cartilage-derived growth factor by heparin affinity chromatography. J Biol Chem. 1985 Feb 25;260(4):2399–2403. [PubMed] [Google Scholar]
- Thomas K. A., Rios-Candelore M., Giménez-Gallego G., DiSalvo J., Bennett C., Rodkey J., Fitzpatrick S. Pure brain-derived acidic fibroblast growth factor is a potent angiogenic vascular endothelial cell mitogen with sequence homology to interleukin 1. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6409–6413. doi: 10.1073/pnas.82.19.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Fuks Z., Bar-Ner M., Ariav Y., Schirrmacher V. Lymphoma cell-mediated degradation of sulfated proteoglycans in the subendothelial extracellular matrix: relationship to tumor cell metastasis. Cancer Res. 1983 Jun;43(6):2704–2711. [PubMed] [Google Scholar]
- Wadzinski M. G., Folkman J., Sasse J., Devey K., Ingber D., Klagsbrun M. Heparin-binding angiogenesis factors: detection by immunological methods. Clin Physiol Biochem. 1987;5(3-4):200–209. [PubMed] [Google Scholar]