Abstract
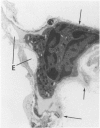
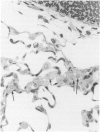
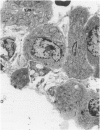
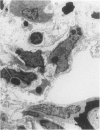
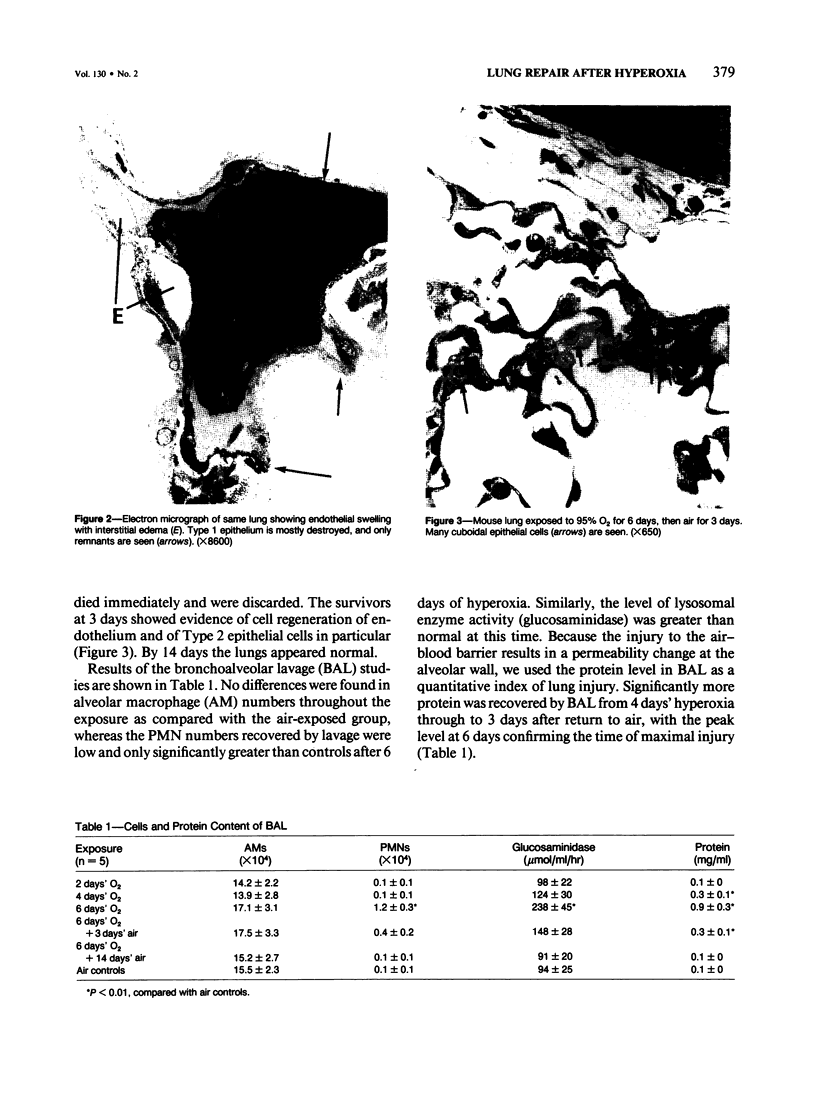
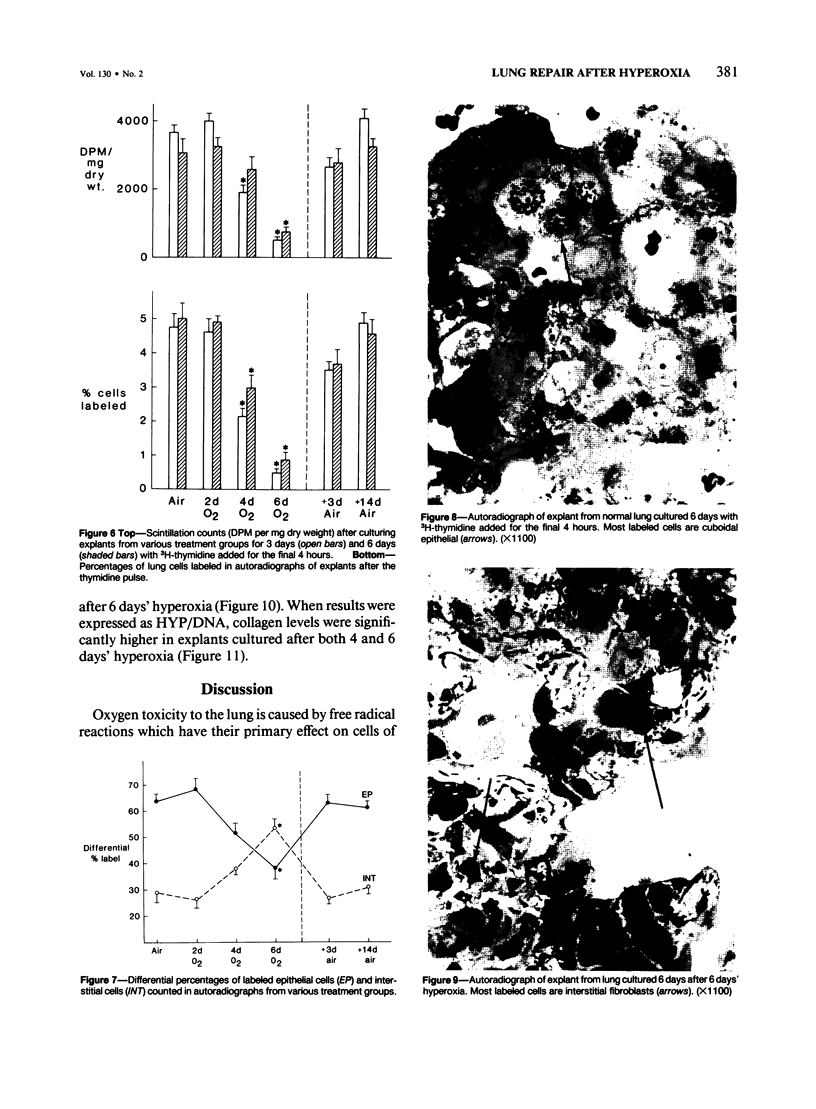
Explants of mouse lung were cultured at various stages of injury after exposure to hyperoxia for determination of whether endothelial or epithelial injury alone could stimulate fibrosis in a blood-free environment. Mice were exposed to 95% O2 for periods up to 6 days. Then one lobe of lung was prepared for organ culture, and others were used for assessment of lung damage by morphologic studies and by the protein and cellular content of bronchoalveolar lavage (BAL) fluid. Explants cultured when the lung showed endothelial injury only were not different from air-exposed controls. As alveolar damage, particularly to Type 1 epithelial cells, increased at 6 days, more protein was found by lavage; and after culture, overall DNA synthesis in explants was reduced. Autoradiography showed that epithelial cell proliferation was preferentially retarded while fibroblast growth became predominant. Collagen production was also significantly increased after 3 and 6 days of culture. In these explants there were few macrophages and no white blood cells or other blood components. Some mice, returned to air after hyperoxia, showed prompt epithelial repair, and cultures of these lungs were not different from controls. The results suggest that severe injury and retarded repair of the alveolar epithelium disturbs normal epithelial-fibroblast interactions and is sufficient to promote the fibrotic process. Less severe injury involving the endothelium only is not associated with fibrosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974 Jan;30(1):35–42. [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H., Wyatt J. P. Oxygen poisoning in mice. Ultrastructural and surfactant studies during exposure and recovery. Arch Pathol. 1970 Nov;90(5):463–472. [PubMed] [Google Scholar]
- Adamson I. Y., King G. M. Sex differences in development of fetal rat lung. II. Quantitative morphology of epithelial-mesenchymal interactions. Lab Invest. 1984 Apr;50(4):461–468. [PubMed] [Google Scholar]
- Bitterman P. B., Adelberg S., Crystal R. G. Mechanisms of pulmonary fibrosis. Spontaneous release of the alveolar macrophage-derived growth factor in the interstitial lung disorders. J Clin Invest. 1983 Nov;72(5):1801–1813. doi: 10.1172/JCI111140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann H. B., Lockwood T., Morgan H. R. Surface biochemical changes accompanying primary infection with Rous sarcoma virus. II. Proteolytic and glycosidase activity and sublethal autolysis. Exp Cell Res. 1974 Jan;83(1):25–30. doi: 10.1016/0014-4827(74)90683-1. [DOI] [PubMed] [Google Scholar]
- Brody A. R., Soler P., Basset F., Haschek W. M., Witschi H. Epithelial-mesenchymal associations of cells in human pulmonary fibrosis and in BHT-oxygen-induced fibrosis in mice. Exp Lung Res. 1981 Aug;2(3):207–220. doi: 10.3109/01902148109052316. [DOI] [PubMed] [Google Scholar]
- Brody J. S., Kaplan N. B. Proliferation of alveolar interstitial cells during postnatal lung growth. Evidence for two distinct populations of pulmonary fibroblasts. Am Rev Respir Dis. 1983 Jun;127(6):763–770. doi: 10.1164/arrd.1983.127.6.763. [DOI] [PubMed] [Google Scholar]
- Goldstein R. H., Fine A. Fibrotic reactions in the lung: the activation of the lung fibroblast. Exp Lung Res. 1986;11(4):245–261. doi: 10.3109/01902148609062828. [DOI] [PubMed] [Google Scholar]
- Haschek W. M., Witschi H. Pulmonary fibrosis--a possible mechanism. Toxicol Appl Pharmacol. 1979 Dec;51(3):475–487. doi: 10.1016/0041-008x(79)90372-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nash G., Blennerhassett J. B., Pontoppidan H. Pulmonary lesions associated with oxygen therapy and artifical ventilation. N Engl J Med. 1967 Feb 16;276(7):368–374. doi: 10.1056/NEJM196702162760702. [DOI] [PubMed] [Google Scholar]
- Reiser K. M., Last J. A. Early cellular events in pulmonary fibrosis. Exp Lung Res. 1986;10(4):331–355. doi: 10.3109/01902148609058286. [DOI] [PubMed] [Google Scholar]
- Smith B. T. Lung maturation in the fetal rat: acceleration by injection of fibroblast-pneumonocyte factor. Science. 1979 Jun 8;204(4397):1094–1095. doi: 10.1126/science.582216. [DOI] [PubMed] [Google Scholar]
- Tate R. M., Repine J. E. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis. 1983 Sep;128(3):552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- Terzaghi M., Nettesheim P., Williams M. L. Repopulation of denuded tracheal grafts with normal, preneoplastic, and neoplastic epithelial cell populations. Cancer Res. 1978 Dec;38(12):4546–4553. [PubMed] [Google Scholar]
- Thurlbeck W. M. Morphologic aspects of bronchopulmonary dysplasia. J Pediatr. 1979 Nov;95(5 Pt 2):842–843. doi: 10.1016/s0022-3476(79)80447-3. [DOI] [PubMed] [Google Scholar]