Abstract
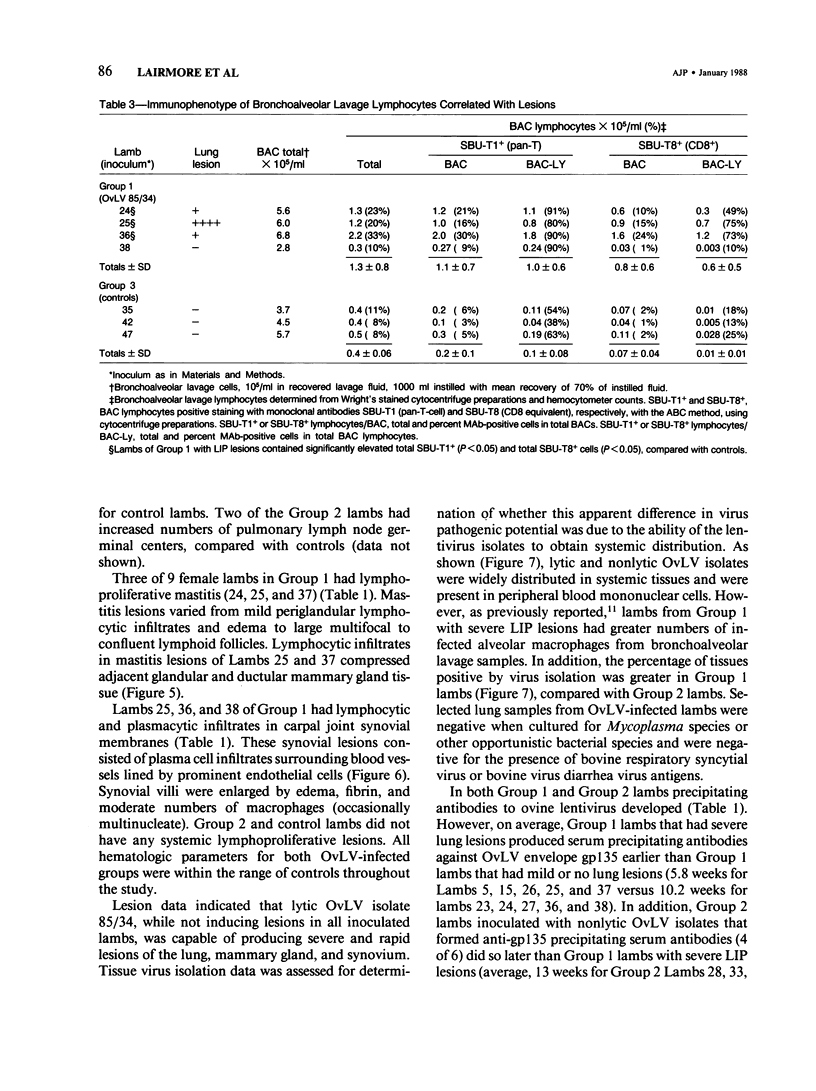
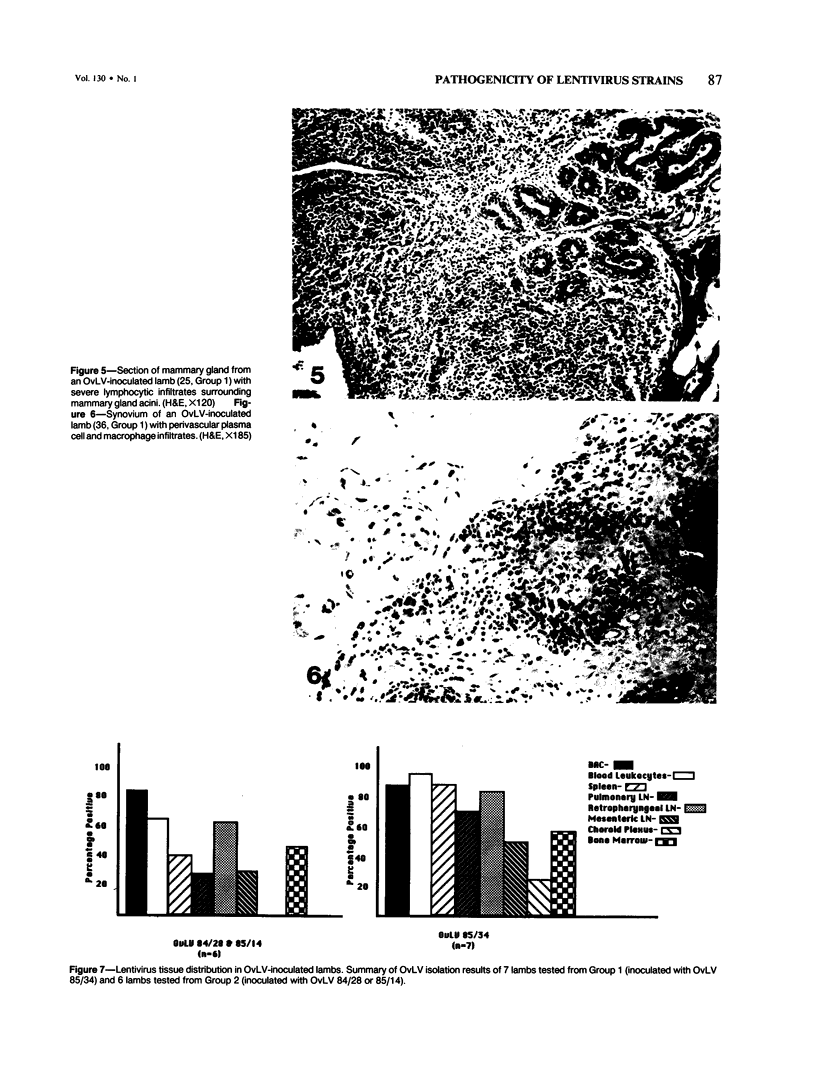
For investigation of the pathogenicity of lentivirus strains, which have distinctly different cytopathic phenotypes in synovial membrane cell culture, plaque-purified, lytic, and nonlytic ovine lentivirus (OvLV) isolates were inoculated intratracheally into two groups of neonatal lambs. Twelve lambs were inoculated with a lytic OvLV isolate and 3 lambs each with two nonlytic OvLV isolates. Five control lambs were inoculated with either virus-free medium or were left uninoculated. In 8 of 12 lambs inoculated with a lytic OvLV isolate mild to severe lesions of lymphoid interstitial pneumonia (LIP) and pulmonary lymphoid hyperplasia developed, 6 of 12 lambs had lesions of pulmonary lymph node follicular hyperplasia, 3 of 9 female lambs had lesions of lymphoproliferative mastitis, 3 of 10 lambs had lesions of lymphocytic/plasmacytic synovitis, and 3 lambs had no lesions. In 3 of 6 lambs inoculated with nonlytic OvLV isolates only mild LIP lesions developed, without concurrent mammary gland or joint lesions. Bronchoalveolar lavage samples from OvLV-diseased lambs contained on average 1.5-fold more numbers of total leukocytes, and 4-fold more numbers of lymphocytes, compared with bronchoalveolar lavage samples of normal lambs. Monoclonal antibodies to ovine lymphocyte surface markers showed that the SBU-T8+ lymphocyte (CD 8 equivalent) was the predominant lymphocyte subset (mean of 65% of total lavaged lymphocytes) in bronchoalveolar lavage samples of 3 diseased lambs. Ovine lentivirus was reisolated from multiple tissues of both groups of OvLV-inoculated lambs, but the percentage of individual tissues infected was greater in lambs inoculated with the lytic viral isolate. Control lambs had no lesions and failed to produce OvLV-specific antibodies or yield OvLV from tissues. All OvLV-inoculated lambs produced either low or undetectable serum virus neutralizing antibodies. In contrast, lambs inoculated with either lytic or nonlytic OvLV produced precipitating antibodies to OvLV glycoprotein and group-specific protein. However, initial detection of precipitating antibodies to OvLV glycoprotein was earlier (mean, 5.8 weeks after inoculation) in OvLV-infected lambs in which severe lymphoproliferative disease developed and delayed (mean, 10.2 weeks after inoculation) in OvLV-infected lambs with mild or no lesions. Together, these results suggest that lentivirus isolates produced disease in a virus strain-dependent manner and suggest that humoral immune responses against OvLV failed to prevent lesion development in lentivirus-infected lambs.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






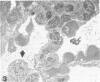
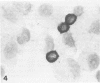
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andiman W. A., Eastman R., Martin K., Katz B. Z., Rubinstein A., Pitt J., Pahwa S., Miller G. Opportunistic lymphoproliferations associated with Epstein-Barr viral DNA in infants and children with AIDS. Lancet. 1985 Dec 21;2(8469-70):1390–1393. doi: 10.1016/s0140-6736(85)92557-7. [DOI] [PubMed] [Google Scholar]
- Chayt K. J., Harper M. E., Marselle L. M., Lewin E. B., Rose R. M., Oleske J. M., Epstein L. G., Wong-Staal F., Gallo R. C. Detection of HTLV-III RNA in lungs of patients with AIDS and pulmonary involvement. JAMA. 1986 Nov 7;256(17):2356–2359. [PubMed] [Google Scholar]
- Cutlip R. C., Jackson T. A., Laird G. A. Immunodiffusion test for ovine progressive pneumonia. Am J Vet Res. 1977 Jul;38(7):1081–1084. [PubMed] [Google Scholar]
- Davis J. L., Molineaux S., Clements J. E. Visna virus exhibits a complex transcriptional pattern: one aspect of gene expression shared with the acquired immunodeficiency syndrome retrovirus. J Virol. 1987 May;61(5):1325–1331. doi: 10.1128/jvi.61.5.1325-1331.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. A., DeMartini J. C. Immunomorphologic and morphometric changes in pulmonary lymph nodes of sheep with progressive pneumonia. Vet Pathol. 1985 Jan;22(1):32–41. doi: 10.1177/030098588502200105. [DOI] [PubMed] [Google Scholar]
- Ellis J. A., DeMartini J. C. Ovine interleukin-2: partial purification and assay in normal sheep and sheep with ovine progressive pneumonia. Vet Immunol Immunopathol. 1985 Jan;8(1-2):15–25. doi: 10.1016/0165-2427(85)90106-0. [DOI] [PubMed] [Google Scholar]
- Ellis T. M., Wilcox G. E., Robinson W. F. Characteristics of cell fusion induced by a caprine retrovirus. Arch Virol. 1985;86(3-4):263–273. doi: 10.1007/BF01309830. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Wong-Staal F., Gallo R. C., Clements J. E., Narayan O., Gilden R. V. Sequence homology and morphologic similarity of HTLV-III and visna virus, a pathogenic lentivirus. Science. 1985 Jan 11;227(4683):173–177. doi: 10.1126/science.2981428. [DOI] [PubMed] [Google Scholar]
- Hess J. L., Clements J. E., Narayan O. cis- and trans-acting transcriptional regulation of visna virus. Science. 1985 Aug 2;229(4712):482–485. doi: 10.1126/science.2990051. [DOI] [PubMed] [Google Scholar]
- Joshi V. V., Kauffman S., Oleske J. M., Fikrig S., Denny T., Gadol C., Lee E. Polyclonal polymorphic B-cell lymphoproliferative disorder with prominent pulmonary involvement in children with acquired immune deficiency syndrome. Cancer. 1987 Apr 15;59(8):1455–1462. doi: 10.1002/1097-0142(19870415)59:8<1455::aid-cncr2820590811>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Joshi V. V., Oleske J. M., Minnefor A. B., Saad S., Klein K. M., Singh R., Zabala M., Dadzie C., Simpser M., Rapkin R. H. Pathologic pulmonary findings in children with the acquired immunodeficiency syndrome: a study of ten cases. Hum Pathol. 1985 Mar;16(3):241–246. doi: 10.1016/s0046-8177(85)80009-5. [DOI] [PubMed] [Google Scholar]
- Joshi V. V., Oleske J. M. Pulmonary lesions in children with the acquired immunodeficiency syndrome: a reappraisal based on data in additional cases and follow-up study of previously reported cases. Hum Pathol. 1986 Jun;17(6):641–642. doi: 10.1016/s0046-8177(86)80143-5. [DOI] [PubMed] [Google Scholar]
- Kennedy-Stoskopf S., Narayan O. Neutralizing antibodies to visna lentivirus: mechanism of action and possible role in virus persistence. J Virol. 1986 Jul;59(1):37–44. doi: 10.1128/jvi.59.1.37-44.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. R., Martin J., Griffing S., Nathanson N., Gorham J., Shen D. T., Petursson G., Georgsson G., Palsson P. A., Lutley R. Precipitating antibodies in experimental visna and natural progressive pneumonia of sheep. Res Vet Sci. 1985 Mar;38(2):129–133. [PubMed] [Google Scholar]
- Koyanagi Y., Miles S., Mitsuyasu R. T., Merrill J. E., Vinters H. V., Chen I. S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987 May 15;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Lairmore M. D., Rosadio R. H., DeMartini J. C. Ovine lentivirus lymphoid interstitial pneumonia. Rapid induction in neonatal lambs. Am J Pathol. 1986 Oct;125(1):173–181. [PMC free article] [PubMed] [Google Scholar]
- Mackay C. R., Maddox J. F., Gogolin-Ewens K. J., Brandon M. R. Characterization of two sheep lymphocyte differentiation antigens, SBU-T1 and SBU-T6. Immunology. 1985 Aug;55(4):729–737. [PMC free article] [PubMed] [Google Scholar]
- Maddox J. F., Mackay C. R., Brandon M. R. Surface antigens, SBU-T4 and SBU-T8, of sheep T lymphocyte subsets defined by monoclonal antibodies. Immunology. 1985 Aug;55(4):739–748. [PMC free article] [PubMed] [Google Scholar]
- Pahwa S., Fikrig S., Kaplan M., Kahn E., Pahwa R. Expressions of HTLV-III infection in a pediatric population. Adv Exp Med Biol. 1985;187:45–51. doi: 10.1007/978-1-4615-9430-7_4. [DOI] [PubMed] [Google Scholar]
- Porterfield J. S. Antibody enhanced viral growth in macrophages. Immunol Lett. 1985;11(3-4):213–217. doi: 10.1016/0165-2478(85)90170-1. [DOI] [PubMed] [Google Scholar]
- Puri N. K., Mackay C. R., Brandon M. R. Sheep lymphocyte antigens (OLA). II. Major histocompatibility complex class II molecules. Immunology. 1985 Dec;56(4):725–733. [PMC free article] [PubMed] [Google Scholar]
- Quérat G., Barban V., Sauze N., Filippi P., Vigne R., Russo P., Vitu C. Highly lytic and persistent lentiviruses naturally present in sheep with progressive pneumonia are genetically distinct. J Virol. 1984 Nov;52(2):672–679. doi: 10.1128/jvi.52.2.672-679.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringler D. J., Hancock W. W., King N. W., Letvin N. L., Daniel M. D., Desrosiers R. C., Murphy G. F. Immunophenotypic characterization of the cutaneous exanthem of SIV-infected rhesus monkeys. Apposition of degenerative Langerhans cells and cytotoxic lymphocytes during the development of acquired immunodeficiency syndrome. Am J Pathol. 1987 Feb;126(2):199–207. [PMC free article] [PubMed] [Google Scholar]
- Sonigo P., Alizon M., Staskus K., Klatzmann D., Cole S., Danos O., Retzel E., Tiollais P., Haase A., Wain-Hobson S. Nucleotide sequence of the visna lentivirus: relationship to the AIDS virus. Cell. 1985 Aug;42(1):369–382. doi: 10.1016/s0092-8674(85)80132-x. [DOI] [PubMed] [Google Scholar]
- Stowring L., Haase A. T., Petursson G., Georgsson G., Palsson P., Lutley R., Roos R., Szuchet S. Detection of visna virus antigens and RNA in glial cells in foci of demyelination. Virology. 1985 Mar;141(2):311–318. doi: 10.1016/0042-6822(85)90264-8. [DOI] [PubMed] [Google Scholar]
- Walker C. M., Moody D. J., Stites D. P., Levy J. A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986 Dec 19;234(4783):1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]