Abstract
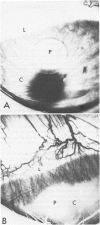
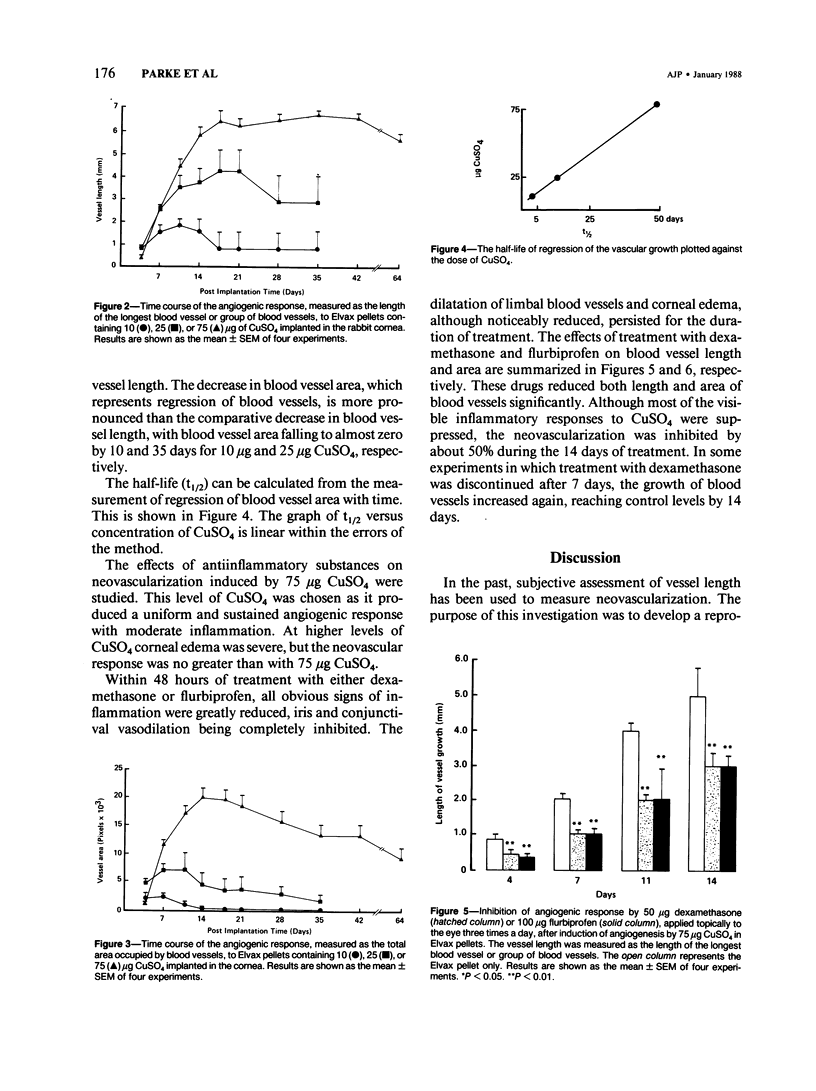
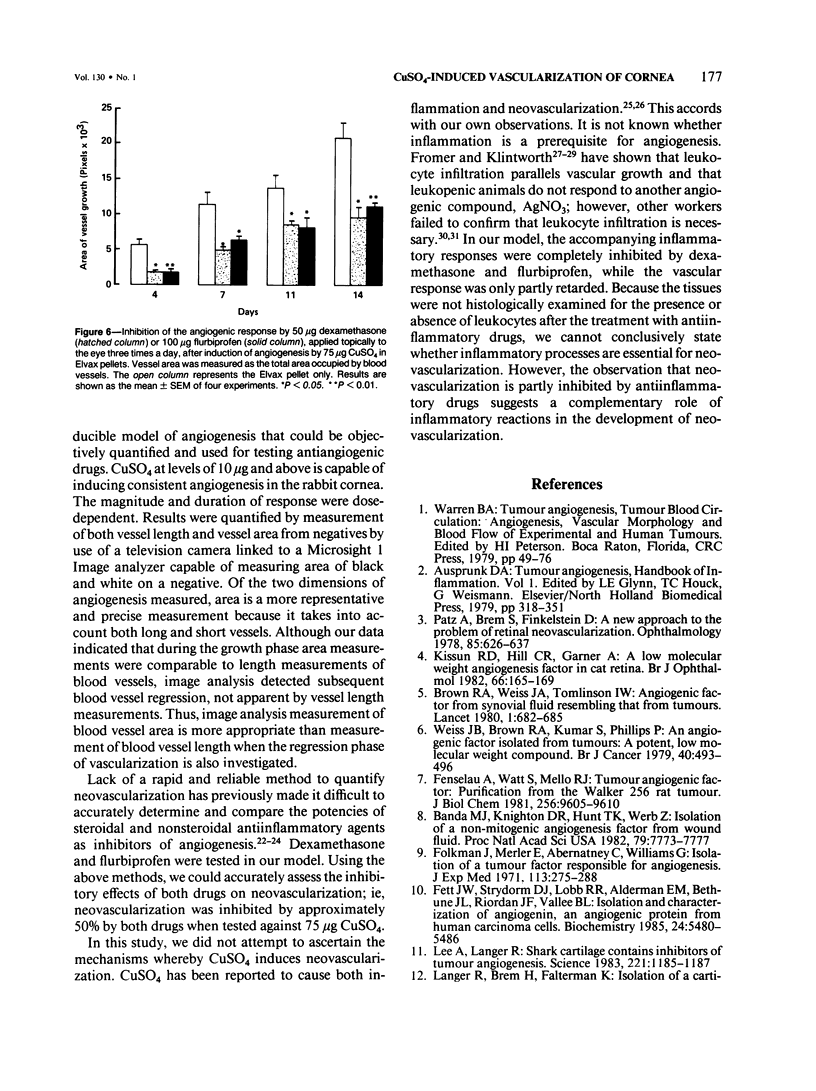
A model of angiogenesis in the rabbit cornea, with reproducible onset and duration of responses, was developed by using CuSO4 as the angiogenic stimulus. The vascularization of the cornea was quantified by means of an image analyzer. In addition, the effects of antiinflammatory compounds, dexamethasone and flurbiprofen, on the angiogenic response to CuSO4 were examined. Elvax pellets containing 10-75 micrograms of CuSO4 implanted in the corneal stroma dose-dependently induced neovascularization, which persisted for more than 64 days at the highest dose. Manual measurements of blood vessel lengths and image analysis measurements of blood vessel areas were comparable during the growth phase of vascularization, but only the image analysis measurements detected a subsequent regression phase. Therefore, the length method of measurement is only useful during the growth phase, whereas the image analysis method is useful during both the growth and regression phases of vascularization. Dexamethasone (50 micrograms, applied topically, three times a day) and flurbiprofen (100 micrograms, applied topically, three times a day) suppressed the inflammation produced by corneal implants containing 75 micrograms CuSO4. However, each drug only inhibited vascular growth by 50% during the 14 days of treatment. Although CuSO4 is not an endogenous angiogenic factor, the model presented in this report may be useful in quantitative evaluation of anti-angiogenic agents.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banda M. J., Knighton D. R., Hunt T. K., Werb Z. Isolation of a nonmitogenic angiogenesis factor from wound fluid. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7773–7777. doi: 10.1073/pnas.79.24.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem H., Folkman J. Inhibition of tumor angiogenesis mediated by cartilage. J Exp Med. 1975 Feb 1;141(2):427–439. doi: 10.1084/jem.141.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. A., Weiss J. B., Tomlinson I. W., Phillips P., Kumar S. Angiogenic factor from synovial fluid resembling that from tumours. Lancet. 1980 Mar 29;1(8170):682–685. [PubMed] [Google Scholar]
- Cooper C. A., Bergamini M. V., Leopold I. H. Use of flurbiprofen to inhibit corneal neovascularization. Arch Ophthalmol. 1980 Jun;98(6):1102–1105. doi: 10.1001/archopht.1980.01020031092017. [DOI] [PubMed] [Google Scholar]
- Deutsch T. A., Hughes W. F. Suppressive effects of indomethacin on thermally induced neovascularization of rabbit corneas. Am J Ophthalmol. 1979 Apr;87(4):536–540. doi: 10.1016/0002-9394(79)90245-9. [DOI] [PubMed] [Google Scholar]
- Eisenstein R., Goren S. B., Shumacher B., Choromokos E. The inhibition of corneal vascularization with aortic extracts in rabbits. Am J Ophthalmol. 1979 Dec;88(6):1005–1012. doi: 10.1016/0002-9394(79)90406-9. [DOI] [PubMed] [Google Scholar]
- Eliason J. A. Leukocytes and experimental corneal vascularization. Invest Ophthalmol Vis Sci. 1978 Nov;17(11):1087–1095. [PubMed] [Google Scholar]
- Esch F., Baird A., Ling N., Ueno N., Hill F., Denoroy L., Klepper R., Gospodarowicz D., Böhlen P., Guillemin R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6507–6511. doi: 10.1073/pnas.82.19.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenselau A., Watt S., Mello R. J. Tumor angiogenic factor. Purification from the Walker 256 rat tumor. J Biol Chem. 1981 Sep 25;256(18):9605–9611. [PubMed] [Google Scholar]
- Fett J. W., Strydom D. J., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985 Sep 24;24(20):5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Folkman J., Merler E., Abernathy C., Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971 Feb 1;133(2):275–288. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromer C. H., Klintworth G. K. An evaluation of the role of leukocytes in the pathogenesis of experimentally induced corneal vascularization. II. Studies on the effect of leukocytic elimination on corneal vascularization. Am J Pathol. 1975 Dec;81(3):531–544. [PMC free article] [PubMed] [Google Scholar]
- Fromer C. H., Klintworth G. K. An evaluation of the role of leukocytes in the pathogenesis of experimentally induced corneal vascularization. III. Studies related to the vasoproliferative capability of polymorphonuclear leukocytes and lymphocytes. Am J Pathol. 1976 Jan;82(1):157–170. [PMC free article] [PubMed] [Google Scholar]
- Fromer C. H., Klintworth G. K. An evaluation of the role of leukocytes in the pathogenesis of experimentally induced corneal vascularization. Am J Pathol. 1975 Jun;79(3):537–554. [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Leapman S. B., Folkman J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J Natl Cancer Inst. 1974 Feb;52(2):413–427. doi: 10.1093/jnci/52.2.413. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Bialecki H., Thakral T. K. The angiogenic activity of the fibroblast and epidermal growth factor. Exp Eye Res. 1979 May;28(5):501–514. doi: 10.1016/0014-4835(79)90038-1. [DOI] [PubMed] [Google Scholar]
- Gross J., Azizkhan R. G., Biswas C., Bruns R. R., Hsieh D. S., Folkman J. Inhibition of tumor growth, vascularization, and collagenolysis in the rabbit cornea by medroxyprogesterone. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1176–1180. doi: 10.1073/pnas.78.2.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey P. T., Cherry P. M. Indomethacin v. dexamethasone in the suppression of corneal neovascularization. Can J Ophthalmol. 1983 Oct;18(6):293–295. [PubMed] [Google Scholar]
- Kissun R. D., Hill C. R., Garner A., Phillips P., Kumar S., Weiss J. B. A low-molecular-weight angiogenic factor in cat retina. Br J Ophthalmol. 1982 Mar;66(3):165–169. doi: 10.1136/bjo.66.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R., Brem H., Falterman K., Klein M., Folkman J. Isolations of a cartilage factor that inhibits tumor neovascularization. Science. 1976 Jul 2;193(4247):70–72. doi: 10.1126/science.935859. [DOI] [PubMed] [Google Scholar]
- Langer R., Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature. 1976 Oct 28;263(5580):797–800. doi: 10.1038/263797a0. [DOI] [PubMed] [Google Scholar]
- Lee A., Langer R. Shark cartilage contains inhibitors of tumor angiogenesis. Science. 1983 Sep 16;221(4616):1185–1187. doi: 10.1126/science.6193581. [DOI] [PubMed] [Google Scholar]
- McAuslan B. R., Gole G. A. Cellular and molecular mechanisms in angiogenesis. Trans Ophthalmol Soc U K. 1980 Sep;100(3):354–358. [PubMed] [Google Scholar]
- Patz A., Brem S., Finkelstein D., Chen C. H., Lutty G., Bennett A., Coughlin W. R., Gardner J. A new approach to the problem of retinal neovascularization. Ophthalmology. 1978 Jun;85(6):626–637. doi: 10.1016/s0161-6420(78)35640-2. [DOI] [PubMed] [Google Scholar]
- Preis I., Langer R., Brem H., Folkman J. Inhibition of neovascularization by an extract derived from vitreous. Am J Ophthalmol. 1977 Sep;84(3):323–328. doi: 10.1016/0002-9394(77)90672-9. [DOI] [PubMed] [Google Scholar]
- Taylor S., Folkman J. Protamine is an inhibitor of angiogenesis. Nature. 1982 May 27;297(5864):307–312. doi: 10.1038/297307a0. [DOI] [PubMed] [Google Scholar]
- Weiss J. B., Brown R. A., Kumar S., Phillips P. An angiogenic factor isolated from tumours: a potent low-molecular-weight compound. Br J Cancer. 1979 Sep;40(3):493–496. doi: 10.1038/bjc.1979.206. [DOI] [PMC free article] [PubMed] [Google Scholar]