Abstract
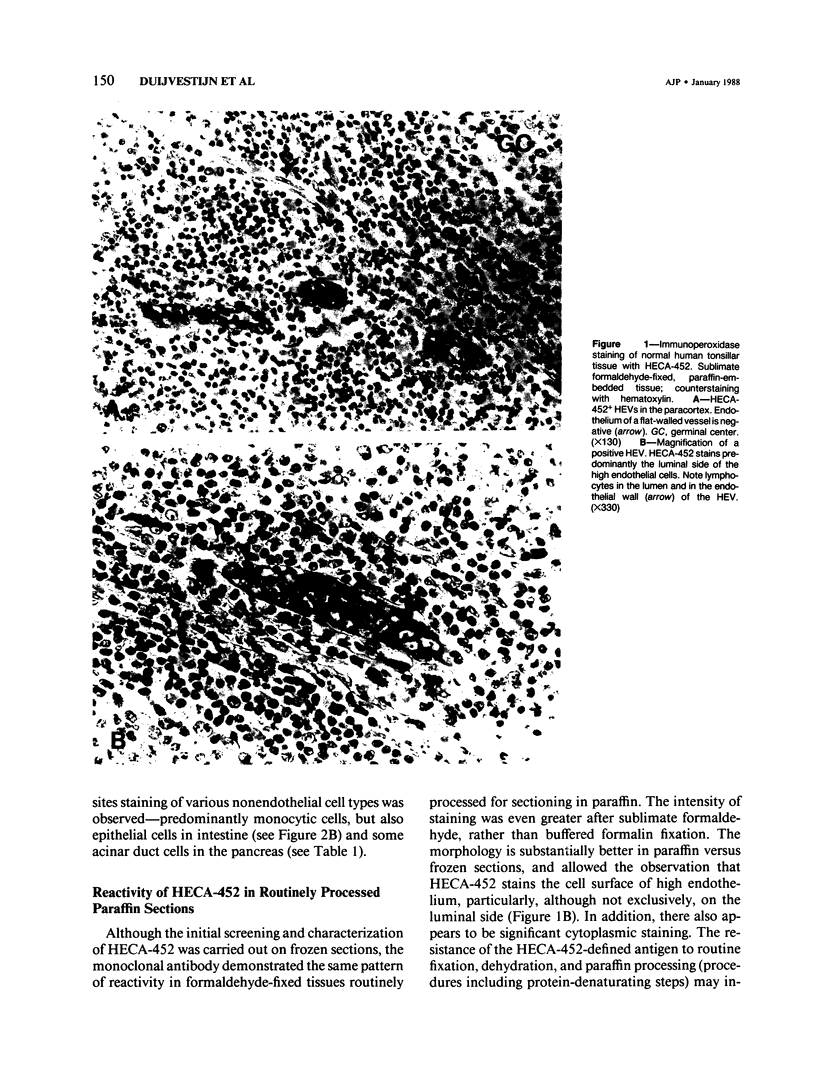
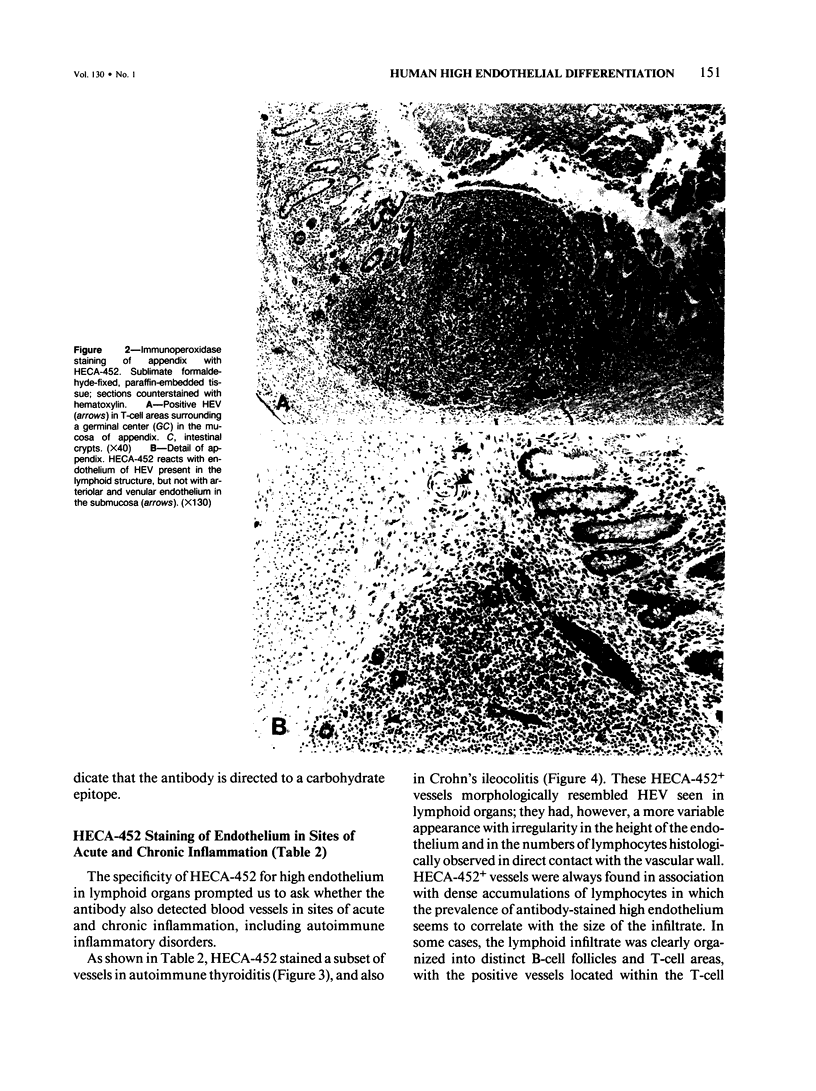
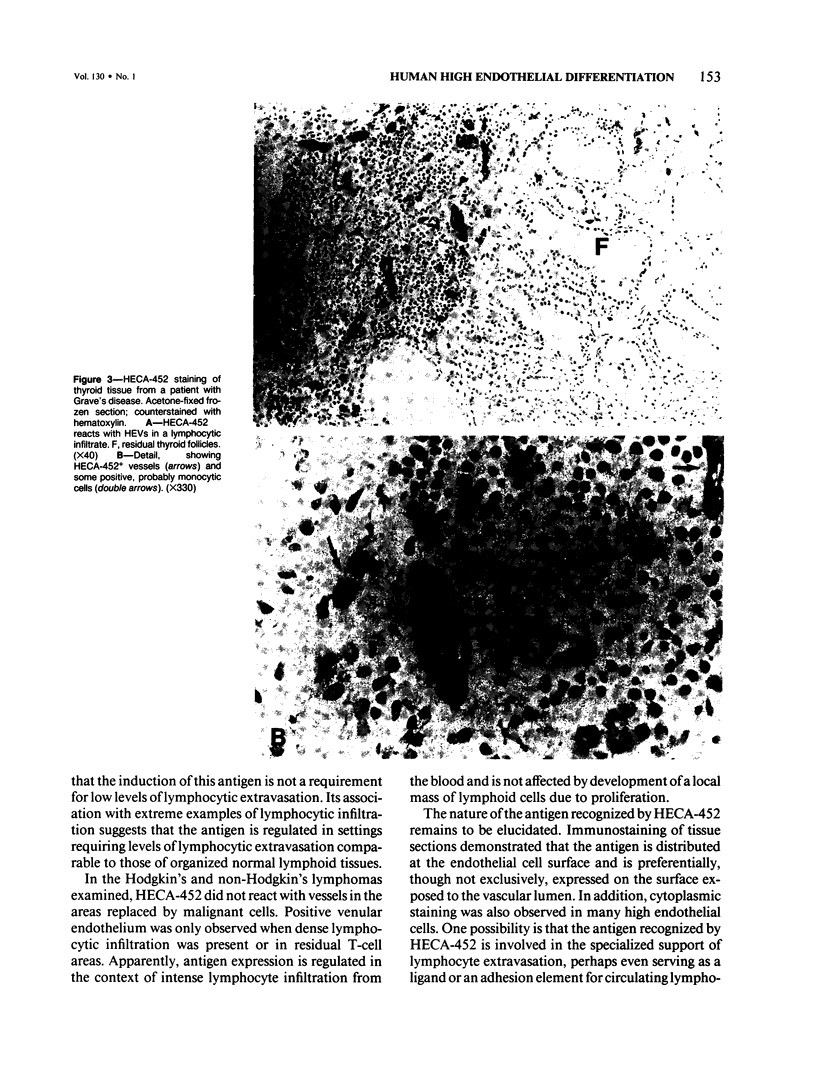
Lymphocyte traffic into lymph nodes and into mucosa-associated lymphoid tissues is regulated by specialized postcapillary high endothelial venules (HEVs). The authors have produced a rat monoclonal antibody, HECA-452, that detects a human endothelial cell differentiation antigen selectively expressed on high endothelium. In immunoperoxidase studies, HECA-452 intensely stains all HEVs within lymphoid organs. In normal nonlymphoid tissues the antibody stains no vascular endothelium. The antibody, in addition to reacting with high endothelium, cross-reacts with a set of monocytic cells. In pathologic states such as autoimmune thyroiditis and Crohn's disease, known for the development of dense, frequently organized, lymphocytic infiltrates, HECA-452 detects HEV-like vessels containing luminal and intramural lymphocytes, presumably in the process of extravasating. The antigen was not expressed at detectable levels by venules in less heavily infiltrated chronic inflammatory sites nor in acutely inflamed tissues. In lymphoid malignancies, the only vessels stained were morphologically characteristic HEVs in association with areas of residual normal lymphoid tissue or reactive lymphocytic infiltrates. The specificity of HECA-452 for high endothelial cells confirms the highly specialized nature of these vessels and will permit studies of the regulation of high endothelial cell differentiation in vivo and in vitro. The HECA-452 antigen is preserved in paraffin sections of sublimate formaldehyde- and also routinely formalin-fixed tissues. Thus, HECA-452 will be widely applicable for the immunohistologic detection of endothelium specialized for the support of highly increased lymphocyte extravasation in inflammatory sites.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. O., Anderson N. D. Lymphocyte emigration from high endothelial venules in rat lymph nodes. Immunology. 1976 Nov;31(5):731–748. [PMC free article] [PubMed] [Google Scholar]
- Bosman F. T., Lindeman J., Kuiper G., van der Wal A., Kreunig J. The influence of fixation on immunoperoxidase staining of plasmacells in paraffin sections of intestinal biopsy specimens. Histochemistry. 1977 Jul 18;53(1):57–62. doi: 10.1007/BF00511210. [DOI] [PubMed] [Google Scholar]
- Butcher E. C., Rouse R. V., Coffman R. L., Nottenburg C. N., Hardy R. R., Weissman I. L. Surface phenotype of Peyer's patch germinal center cells: implications for the role of germinal centers in B cell differentiation. J Immunol. 1982 Dec;129(6):2698–2707. [PubMed] [Google Scholar]
- Butcher E. C., Scollay R. G., Weissman I. L. Lymphocyte adherence to high endothelial venules: characterization of a modified in vitro assay, and examination of the binding of syngeneic and allogeneic lymphocyte populations. J Immunol. 1979 Nov;123(5):1996–2003. [PubMed] [Google Scholar]
- Butcher E. C., Scollay R. G., Weissman I. L. Organ specificity of lymphocyte migration: mediation by highly selective lymphocyte interaction with organ-specific determinants on high endothelial venules. Eur J Immunol. 1980 Jul;10(7):556–561. doi: 10.1002/eji.1830100713. [DOI] [PubMed] [Google Scholar]
- Duijvestijn A. M., Kerkhove M., Bargatze R. F., Butcher E. C. Lymphoid tissue- and inflammation-specific endothelial cell differentiation defined by monoclonal antibodies. J Immunol. 1987 Feb 1;138(3):713–719. [PubMed] [Google Scholar]
- Duijvestijn A. M., Schreiber A. B., Butcher E. C. Interferon-gamma regulates an antigen specific for endothelial cells involved in lymphocyte traffic. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9114–9118. doi: 10.1073/pnas.83.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijvestijn A. M., Schutte R., Köhler Y. G., Korn C., Hoefsmit E. C. Characterization of the population of phagocytic cells in thymic cell suspensions. A morphological and cytochemical study. Cell Tissue Res. 1983;231(2):313–323. doi: 10.1007/BF00222183. [DOI] [PubMed] [Google Scholar]
- Freemont A. J. A possible route for lymphocyte migration into diseased tissues. J Clin Pathol. 1983 Feb;36(2):161–166. doi: 10.1136/jcp.36.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemont A. J., Ford W. L. Functional and morphological changes in post-capillary venules in relation to lymphocytic infiltration into BCG-induced granulomata in rat skin. J Pathol. 1985 Sep;147(1):1–12. doi: 10.1002/path.1711470102. [DOI] [PubMed] [Google Scholar]
- Freemont A. J., Jones C. J., Bromley M., Andrews P. Changes in vascular endothelium related to lymphocyte collections in diseased synovia. Arthritis Rheum. 1983 Dec;26(12):1427–1433. doi: 10.1002/art.1780261203. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Shannon S. L. Peroxidase arthritis. II. Lymphoid cell-endothelial interactions during a developing immunologic inflammatory response. Am J Pathol. 1972 Oct;69(1):7–24. [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The gut-associated lymphoid system: nature and properties of the large dividing cells. Eur J Immunol. 1974 Jun;4(6):435–443. doi: 10.1002/eji.1830040610. [DOI] [PubMed] [Google Scholar]
- Hendriks H. R., Eestermans I. L. Disappearance and reappearance of high endothelial venules and immigrating lymphocytes in lymph nodes deprived of afferent lymphatic vessels: a possible regulatory role of macrophages in lymphocyte migration. Eur J Immunol. 1983 Aug;13(8):663–669. doi: 10.1002/eji.1830130811. [DOI] [PubMed] [Google Scholar]
- Jalkanen S. T., Bargatze R. F., Herron L. R., Butcher E. C. A lymphoid cell surface glycoprotein involved in endothelial cell recognition and lymphocyte homing in man. Eur J Immunol. 1986 Oct;16(10):1195–1202. doi: 10.1002/eji.1830161003. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Steere A. C., Fox R. I., Butcher E. C. A distinct endothelial cell recognition system that controls lymphocyte traffic into inflamed synovium. Science. 1986 Aug 1;233(4763):556–558. doi: 10.1126/science.3726548. [DOI] [PubMed] [Google Scholar]
- Katz M. E., Tite J. P., Janeway C. A., Jr The immunobiology of T cell responses to Mls-locus-disparate stimulator cells. III. Helper and cytolytic functions of cloned, Mls-reactive T cell lines. J Immunol. 1986 Jan;136(1):1–5. [PubMed] [Google Scholar]
- Knowles D. M., 2nd, Tolidjian B., Marboe C., D'Agati V., Grimes M., Chess L. Monoclonal anti-human monocyte antibodies OKM1 and OKM5 possess distinctive tissue distributions including differential reactivity with vascular endothelium. J Immunol. 1984 May;132(5):2170–2173. [PubMed] [Google Scholar]
- Kraal G., Duijvestijn A. M., Hendriks H. H. The endothelium of the high endothelial venule: a specialized endothelium with unique properties. Exp Cell Biol. 1987;55(1):1–10. doi: 10.1159/000163388. [DOI] [PubMed] [Google Scholar]
- Kraal G., Weissman I. L., Butcher E. C. Differences in in vivo distribution and homing of T cell subsets to mucosal vs nonmucosal lymphoid organs. J Immunol. 1983 Mar;130(3):1097–1102. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Pals S. T., Kraal G., Horst E., de Groot A., Scheper R. J., Meijer C. J. Human lymphocyte-high endothelial venule interaction: organ-selective binding of T and B lymphocyte populations to high endothelium. J Immunol. 1986 Aug 1;137(3):760–763. [PubMed] [Google Scholar]
- Rasmussen R. A., Chin Y. H., Woodruff J. J., Easton T. G. Lymphocyte recognition of lymph node high endothelium. VII. Cell surface proteins involved in adhesion defined by monoclonal anti-HEBFLN (A.11) antibody. J Immunol. 1985 Jul;135(1):19–24. [PubMed] [Google Scholar]
- Rosen S. D., Singer M. S., Yednock T. A., Stoolman L. M. Involvement of sialic acid on endothelial cells in organ-specific lymphocyte recirculation. Science. 1985 May 24;228(4702):1005–1007. doi: 10.1126/science.4001928. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Smith M. E., Ford W. L. The recirculating lymphocyte pool of the rat: a systematic description of the migratory behaviour of recirculating lymphocytes. Immunology. 1983 May;49(1):83–94. [PMC free article] [PubMed] [Google Scholar]
- Stamper H. B., Jr, Woodruff J. J. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976 Sep 1;144(3):828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens S. K., Weissman I. L., Butcher E. C. Differences in the migration of B and T lymphocytes: organ-selective localization in vivo and the role of lymphocyte-endothelial cell recognition. J Immunol. 1982 Feb;128(2):844–851. [PubMed] [Google Scholar]
- Stoolman L. M., Tenforde T. S., Rosen S. D. Phosphomannosyl receptors may participate in the adhesive interaction between lymphocytes and high endothelial venules. J Cell Biol. 1984 Oct;99(4 Pt 1):1535–1540. doi: 10.1083/jcb.99.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vliet E., Melis M., Van Ewijk W. Monoclonal antibodies to stromal cell types of the mouse thymus. Eur J Immunol. 1984 Jun;14(6):524–529. doi: 10.1002/eji.1830140608. [DOI] [PubMed] [Google Scholar]