Abstract
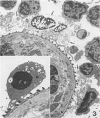
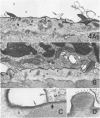
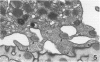
In this study was examined the interaction between schistosomula of Schistosoma mansoni and human monocyte-derived macrophages activated with interferon gamma (IFN-gamma). Peripheral blood monocytes were matured for 6 days and activated by further culture with IFN-gamma (600 U/ml). These IFN-gamma-treated monocyte-derived macrophages are cytotoxic for the tumor cell line K562, which is not killed by nonactivated monocyte-derived macrophages. Activated monocyte-derived macrophages were incubated with schistosomula at ratios of 10(3):1 and 10(4):1 in the presence of serum pooled from patients with schistosomiasis. This antiserum promoted an increased adherence of cells to the parasite. However, the activated monocyte-derived macrophages failed to kill the schistosomula under all conditions tested. On the contrary, the monocyte-derived macrophages were killed by schistosomula in a time-dependent and antibody-dependent manner, which was most evident at a lower effector/target ratio, 200:1. Electron microscopy showed that monocyte-derived macrophages were lysed on the surface of schistosomula. Further, both monocyte-derived macrophages and contaminating blood platelets fused with the parasite surface membrane, so that the cell plasma membrane and the outer tegumental membrane formed a hybrid membrane. The results indicate that matured human monocyte-derived macrophages activated by IFN-gamma are unable to kill schistosomula. Instead, the effector cells fuse with the parasites and are lysed by them.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar A. R., McKean J. R., Smithers S. R., Kay A. B. Human eosinophil- and neutrophil-mediated killing of schistosomula of Schistosoma mansoni in vitro. I. Enhancement of complement-dependent damage by mast cell-derived mediators and formyl methionyl peptides. J Immunol. 1980 Mar;124(3):1122–1129. [PubMed] [Google Scholar]
- Butterworth A. E., David J. R., Franks D., Mahmoud A. A., David P. H., Sturrock R. F., Houba V. Antibody-dependent eosinophil-mediated damage to 51Cr-labeled schistosomula of Schistosoma mansoni: damage by purieid eosinophils. J Exp Med. 1977 Jan 1;145(1):136–150. doi: 10.1084/jem.145.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. E., Taylor D. W., Veith M. C., Vadas M. A., Dessein A., Sturrock R. F., Wells E. Studies on the mechanisms of immunity in human schistosomiasis. Immunol Rev. 1982;61:5–39. doi: 10.1111/j.1600-065x.1982.tb00372.x. [DOI] [PubMed] [Google Scholar]
- Caulfield J. P., Cianci C. M. Human erythrocytes adhering to schistosomula of Schistosoma mansoni lyse and fail to transfer membrane components to the parasite. J Cell Biol. 1985 Jul;101(1):158–166. doi: 10.1083/jcb.101.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Korman G., Butterworth A. E., Hogan M., David J. R. Partial and complete detachment of neutrophils and eosinophils from schistosomula: evidence for the establishment of continuity between a fused and normal parasite membrane. J Cell Biol. 1980 Jul;86(1):64–76. doi: 10.1083/jcb.86.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Korman G., Butterworth A. E., Hogan M., David J. R. The adherence of human neutrophils and eosinophils to schistosomula: evidence for membrane fusion between cells and parasites. J Cell Biol. 1980 Jul;86(1):46–63. doi: 10.1083/jcb.86.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Lenzi H. L., Elsas P., Dessein A. J. Ultrastructure of the attack of eosinophils stimulated by blood mononuclear cell products on schistosomula of Schistosoma mansoni. Am J Pathol. 1985 Sep;120(3):380–390. [PMC free article] [PubMed] [Google Scholar]
- Clegg J. A., Smithers S. R., Terry R. J. Acquisition of human antigens by Schistosoma mansoni during cultivation in vitro. Nature. 1971 Aug 27;232(5313):653–654. doi: 10.1038/232653a0. [DOI] [PubMed] [Google Scholar]
- Dean D. A., Sell K. W. Surface antigens on Schistosoma mansoni. II. Adsorption of a Forssman-like host antigen by schistosomula. Clin Exp Immunol. 1972 Dec;12(4):525–540. [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Mahmoud A. A. Killing of schistosomula of Schistosoma mansoni by normal human monocytes. J Immunol. 1979 Aug;123(2):949–951. [PubMed] [Google Scholar]
- Fischer D. G., Hubbard W. J., Koren H. S. Tumor cell killing by freshly isolated peripheral blood monocytes. Cell Immunol. 1981 Mar 1;58(2):426–435. doi: 10.1016/0008-8749(81)90235-5. [DOI] [PubMed] [Google Scholar]
- Gemsa D., Debatin K. M., Kramer W., Kubelka C., Deimann W., Kees U., Krammer P. H. Macrophage-activating factors from different T cell clones induce distinct macrophage functions. J Immunol. 1983 Aug;131(2):833–844. [PubMed] [Google Scholar]
- Gitter B. D., McCormick S. L., Damian R. T. Murine alloantigen acquisition by Schistosoma mansoni: presence of H-2K determinants on adult worms and failure of allogeneic lymphocytes to recognize acquired MHC gene products on schistosomula. J Parasitol. 1982 Aug;68(4):513–518. [PubMed] [Google Scholar]
- Glauert A. M., Butterworth A. E., Sturrock R. F., Houba V. The mechansim of antibody-dependent, eosinophil-mediated damage to schistosomula of Schistosoma mansoni in vitro: a study by phase-contrast and electron microscopy. J Cell Sci. 1978 Dec;34:173–192. doi: 10.1242/jcs.34.1.173. [DOI] [PubMed] [Google Scholar]
- Golan D. E., Brown C. S., Cianci C. M., Furlong S. T., Caulfield J. P. Schistosomula of Schistosoma mansoni use lysophosphatidylcholine to lyse adherent human red blood cells and immobilize red cell membrane components. J Cell Biol. 1986 Sep;103(3):819–828. doi: 10.1083/jcb.103.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. L., Sher A., Lazdins J. K., Meltzer M. S. Macrophages as effector cells of protective immunity in murine schistosomiasis. II. Killing of newly transformed schistosomula in vitro by macrophages activated as a consequence of Schistosoma mansoni infection. J Immunol. 1982 Apr;128(4):1535–1540. [PubMed] [Google Scholar]
- Joseph M., Capron A., Butterworth A. E., Sturrock R. F., Houba V. Cytotoxicity of human and baboon mononuclear phagocytes against schistosomula in vitro: induction by immune complexes containing IgE and Schistosoma mansoni antigens. Clin Exp Immunol. 1978 Jul;33(1):48–56. [PMC free article] [PubMed] [Google Scholar]
- Kleinerman E. S., Schroit A. J., Fogler W. E., Fidler I. J. Tumoricidal activity of human monocytes activated in vitro by free and liposome-encapsulated human lymphokines. J Clin Invest. 1983 Jul;72(1):304–315. doi: 10.1172/JCI110970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubelka C. F., Ruppel A., Krammer P. H., Gemsa D. Killing of schistosomula of Schistosoma mansoni by macrophages: induction by T-cell clone-derived lymphokines and interferon-gamma. Parasitology. 1986 Apr;92(Pt 2):325–336. doi: 10.1017/s003118200006409x. [DOI] [PubMed] [Google Scholar]
- Lazdins J. K., Stein M. J., David J. R., Sher A. Schistosoma mansoni: rapid isolation and purification of schistosomula of different developmental stages by centrifugation on discontinuous density gradients of Percoll. Exp Parasitol. 1982 Feb;53(1):39–44. doi: 10.1016/0014-4894(82)90090-x. [DOI] [PubMed] [Google Scholar]
- Le J., Prensky W., Yip Y. K., Chang Z., Hoffman T., Stevenson H. C., Balazs I., Sadlik J. R., Vilcek J. Activation of human monocyte cytotoxicity by natural and recombinant immune interferon. J Immunol. 1983 Dec;131(6):2821–2826. [PubMed] [Google Scholar]
- Lee J. C., Rebar L., Young P., Ruscetti F. W., Hanna N., Poste G. Identification and characterization of a human T cell line-derived lymphokine with MAF-like activity distinct from interferon-gamma. J Immunol. 1986 Feb 15;136(4):1322–1328. [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S., Peters P. A. A role for the eosinophil in acquired resistance to Schistosoma mansoni infection as determined by antieosinophil serum. J Exp Med. 1975 Oct 1;142(4):805–813. doi: 10.1084/jem.142.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds G. R., Ellner J. J., el-Kholy A., Mahmoud A. A. Monocyte-mediated killing of schistosomula of Schistosoma mansoni: alterations in human Schistosomiasis mansoni and tuberculosis. J Immunol. 1981 Oct;127(4):1538–1542. [PubMed] [Google Scholar]
- Olds G. R., Kholy A. E., Ellner J. J. Two distinctive patterns of monocyte immunoregulatory and effector functions in heavy human infections with Schistosoma mansoni. J Immunol. 1983 Aug;131(2):954–958. [PubMed] [Google Scholar]
- Pearce E. J., Basch P. F., Sher A. Evidence that the reduced surface antigenicity of developing Schistosoma mansoni schistosomula is due to antigen shedding rather than host molecule acquisition. Parasite Immunol. 1986 Jan;8(1):79–94. doi: 10.1111/j.1365-3024.1986.tb00835.x. [DOI] [PubMed] [Google Scholar]
- Ramalho-Pinto F. J., Gazzinelli G., Howells R. E., Mota-Santos T. A., Figueiredo E. A., Pellegrino J. Schistosoma mansoni: defined system for stepwise transformation of cercaria to schistosomule in vitro. Exp Parasitol. 1974 Dec;36(3):360–372. doi: 10.1016/0014-4894(74)90076-9. [DOI] [PubMed] [Google Scholar]
- STIREWALT M. A. The influence of previous infection of mice with Schistosoma mansoni on a challenging infection with the homologous parasite. Am J Trop Med Hyg. 1953 Sep;2(5):867–882. doi: 10.4269/ajtmh.1953.2.867. [DOI] [PubMed] [Google Scholar]
- Samuelson J. C., Caulfield J. P., David J. R. Schistosomula of Schistosoma mansoni clear concanavalin A from their surface by sloughing. J Cell Biol. 1982 Aug;94(2):355–362. doi: 10.1083/jcb.94.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson J. C., Caulfield J. P. Loss of covalently labeled glycoproteins and glycolipids from the surface of newly transformed schistosomula of Schistosoma mansoni. J Cell Biol. 1982 Aug;94(2):363–369. doi: 10.1083/jcb.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Hall B. F., Vadas M. A. Acquisition of murine major histocompatibility complex gene products by schistosomula of Schistosoma mansoni. J Exp Med. 1978 Jul 1;148(1):46–57. doi: 10.1084/jem.148.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Sacks D. L., Simpson A. J., Singer A. Dichotomy in the tissue origin of schistosome acquired class I and class II major histocompatibility complex antigens. J Exp Med. 1984 Mar 1;159(3):952–957. doi: 10.1084/jem.159.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A. J., Payares G., Walker T., Knight M., Smithers S. R. The modulation of expression of polypeptide surface antigens on developing schistosomula of Schistosoma mansoni. J Immunol. 1984 Nov;133(5):2725–2730. [PubMed] [Google Scholar]
- Simpson A. J., Singer D., McCutchan T. F., Sacks D. L., Sher A. Evidence that schistosome MHC antigens are not synthesized by the parasite but are acquired from the host as intact glycoproteins. J Immunol. 1983 Aug;131(2):962–965. [PubMed] [Google Scholar]
- Smithers S. R., Terry R. J., Hockley D. J. Host antigens in schistosomiasis. Proc R Soc Lond B Biol Sci. 1969 Feb 25;171(1025):483–494. doi: 10.1098/rspb.1969.0007. [DOI] [PubMed] [Google Scholar]
- Vadas M. A., David J. R., Butterworth A., Pisani N. T., Siongok T. A. A new method for the purification of human eosinophils and neutrophils, and a comparison of the ability of these cells to damage schistosomula of Schistosoma mansoni. J Immunol. 1979 Apr;122(4):1228–1236. [PubMed] [Google Scholar]
- Warren K. S. Regulation of the prevalence and intensity of schistosomiasis in man: immunology or ecology? J Infect Dis. 1973 May;127(5):595–609. doi: 10.1093/infdis/127.5.595. [DOI] [PubMed] [Google Scholar]
- Weller P. F., Goetzl E. J., Austen K. F. Identification of human eosinophil lysophospholipase as the constituent of Charcot-Leyden crystals. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7440–7443. doi: 10.1073/pnas.77.12.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]