Abstract
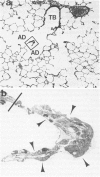
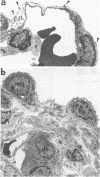
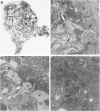
Inhaled chrysotile asbestos fibers have been shown to deposit initially on the first alveolar duct bifurcations. In brief accidental exposure to asbestos, this would be the most likely site of a significant cellular or fibrotic reaction. The characteristics and progression of tissue reactions occurring at first alveolar duct bifurcations after a single brief asbestos exposure was defined using morphometric techniques. Seven-week-old rats were exposed, nose only, for 1 hour to chrysotile asbestos fibers. After the exposure, the animals were kept in air for 2 days or 1 month, and then their lungs were fixed by vascular perfusion or by intratracheal instillation of 2% glutaraldehyde. The first bifurcations of seven alveolar ducts in each animal were isolated from plastic-embedded tissue and thin-sectioned for electron-microscopic analysis. Two days after exposure, the volume of epithelium and interstitium in the duct bifurcations had increased by 78% and 28%, respectively (P less than 0.05). The total number and volume of alveolar macrophages on the bifurcations increased about 10 times (P less than 0.05), whereas the number and volume of interstitial macrophages increased threefold (P less than 0.05). Statistically significant increases in the numbers of Type I (82%) and Type II (29%) epithelial cells also occurred. One month after the 1-hour exposure, the volume of epithelium and the number of Type I and Type II cells were still greater than control values, but these differences no longer achieved statistical significance. The volume of the interstitium, on the other hand, increased 67% (P less than 0.05), and this was accompanied by a persistently high number of interstitial macrophages, accumulation of myofibroblasts/smooth muscle cells, and an increased volume of interstitial matrix. These results demonstrate that a brief exposure to chrysotile asbestos causes a rapid response that involves an influx of macrophages to the first alveolar duct bifurcations and alterations in the alveolar epithelium. These acute structural changes are followed by a progressive response manifested by increased numbers of interstitial cells and localized interstitial fibrosis.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachofen M., Weibel E. R. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis. 1977 Oct;116(4):589–615. doi: 10.1164/arrd.1977.116.4.589. [DOI] [PubMed] [Google Scholar]
- Barry B. E., Miller F. J., Crapo J. D. Effects of inhalation of 0.12 and 0.25 parts per million ozone on the proximal alveolar region of juvenile and adult rats. Lab Invest. 1985 Dec;53(6):692–704. [PubMed] [Google Scholar]
- Barry B. E., Wong K. C., Brody A. R., Crapo J. D. Reaction of rat lungs to inhaled chrysotile asbestos following acute and subchronic exposures. Exp Lung Res. 1983 Jul;5(1):1–21. doi: 10.3109/01902148309061501. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Rennard S. I., Hunninghake G. W., Crystal R. G. Human alveolar macrophage growth factor for fibroblasts. Regulation and partial characterization. J Clin Invest. 1982 Oct;70(4):806–822. doi: 10.1172/JCI110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody A. R., Hill L. H., Adkins B., Jr, O'Connor R. W. Chrysotile asbestos inhalation in rats: deposition pattern and reaction of alveolar epithelium and pulmonary macrophages. Am Rev Respir Dis. 1981 Jun;123(6):670–679. doi: 10.1164/arrd.1981.123.6.670. [DOI] [PubMed] [Google Scholar]
- Brody A. R., Hill L. H. Interstitial accumulation of inhaled chrysotile asbestos fibers and consequent formation of microcalcifications. Am J Pathol. 1982 Oct;109(1):107–114. [PMC free article] [PubMed] [Google Scholar]
- Brody A. R., Roe M. W. Deposition pattern of inorganic particles at the alveolar level in the lungs of rats and mice. Am Rev Respir Dis. 1983 Oct;128(4):724–729. doi: 10.1164/arrd.1983.128.4.724. [DOI] [PubMed] [Google Scholar]
- Bégin R., Rola-Pleszczynski M., Massé S., Lemaire I., Sirois P., Boctor M., Nadeau D., Drapeau G., Bureau M. A. Asbestos-induced lung injury in the sheep model: the initial alveolitis. Environ Res. 1983 Feb;30(1):195–210. doi: 10.1016/0013-9351(83)90180-9. [DOI] [PubMed] [Google Scholar]
- Bégin R., Rola-Pleszczynski M., Sirois P., Lemaire I., Nadeau D., Bureau M. A., Massé S. Early lung events following low-dose asbestos exposure. Environ Res. 1981 Dec;26(2):392–401. doi: 10.1016/0013-9351(81)90215-2. [DOI] [PubMed] [Google Scholar]
- Chang L. Y., Graham J. A., Miller F. J., Ospital J. J., Crapo J. D. Effects of subchronic inhalation of low concentrations of nitrogen dioxide. I. The proximal alveolar region of juvenile and adult rats. Toxicol Appl Pharmacol. 1986 Mar 30;83(1):45–61. doi: 10.1016/0041-008x(86)90321-2. [DOI] [PubMed] [Google Scholar]
- Chang L. Y., Mercer R. R., Crapo J. D. Differential distribution of brush cells in the rat lung. Anat Rec. 1986 Sep;216(1):49–54. doi: 10.1002/ar.1092160109. [DOI] [PubMed] [Google Scholar]
- Craighead J. E., Abraham J. L., Churg A., Green F. H., Kleinerman J., Pratt P. C., Seemayer T. A., Vallyathan V., Weill H. The pathology of asbestos-associated diseases of the lungs and pleural cavities: diagnostic criteria and proposed grading schema. Report of the Pneumoconiosis Committee of the College of American Pathologists and the National Institute for Occupational Safety and Health. Arch Pathol Lab Med. 1982 Oct 8;106(11):544–596. [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Foscue H. A., Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980 Jul;122(1):123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- Davies P., Allison A. C., Ackerman J., Butterfield A., Williams S. Asbestos induces selective release of lysosomal enzymes from mononuclear phagocytes. Nature. 1974 Oct 4;251(5474):423–425. doi: 10.1038/251423a0. [DOI] [PubMed] [Google Scholar]
- Davis J. M., Beckett S. T., Bolton R. E., Collings P., Middleton A. P. Mass and number of fibres in the pathogenesis of asbestos-related lung disease in rats. Br J Cancer. 1978 May;37(5):673–688. doi: 10.1038/bjc.1978.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Dekker N. P., Cabral-Anderson L. J., Shami S. G. Morphological basis of tolerance to ozone. Exp Mol Pathol. 1985 Jun;42(3):366–376. doi: 10.1016/0014-4800(85)90086-3. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Johnson L. V., Stephens R. J., Freeman G. Cell renewal in the lungs of rats exposed to low levels of ozone. Exp Mol Pathol. 1976 Feb;24(1):70–83. doi: 10.1016/0014-4800(76)90058-7. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Stephens R. J., Cabral L. J., Freeman G. Cell renewal in the lungs of rats exposed to low levels of NO2. Arch Environ Health. 1972 Mar;24(3):180–188. doi: 10.1080/00039896.1972.10666067. [DOI] [PubMed] [Google Scholar]
- Harmsen A. G., Muggenburg B. A., Snipes M. B., Bice D. E. The role of macrophages in particle translocation from lungs to lymph nodes. Science. 1985 Dec 13;230(4731):1277–1280. doi: 10.1126/science.4071052. [DOI] [PubMed] [Google Scholar]
- Holt P. F., Mills J., Young D. K. Experimental asbestosis in the guinea-pig. J Pathol Bacteriol. 1966 Jul;92(1):185–195. doi: 10.1002/path.1700920119. [DOI] [PubMed] [Google Scholar]
- Jester J. V., Rodrigues M. M., Herman I. M. Characterization of avascular corneal wound healing fibroblasts. New insights into the myofibroblast. Am J Pathol. 1987 Apr;127(1):140–148. [PMC free article] [PubMed] [Google Scholar]
- Kouzan S., Brody A. R., Nettesheim P., Eling T. Production of arachidonic acid metabolites by macrophages exposed in vitro to asbestos, carbonyl iron particles, or calcium ionophore. Am Rev Respir Dis. 1985 Apr;131(4):624–632. doi: 10.1164/arrd.1985.131.4.624. [DOI] [PubMed] [Google Scholar]
- Leslie C. C., McCormick-Shannon K., Cook J. L., Mason R. J. Macrophages stimulate DNA synthesis in rat alveolar type II cells. Am Rev Respir Dis. 1985 Dec;132(6):1246–1252. doi: 10.1164/arrd.1985.132.6.1246. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Reid L. The alveolar brush cell in rat lung--a third pneumonocyte. J Ultrastruct Res. 1968 Apr;23(1):71–80. doi: 10.1016/s0022-5320(68)80032-2. [DOI] [PubMed] [Google Scholar]
- Pinkerton K. E., Brody A. R., McLaurin D. A., Adkins B., Jr, O'Connor R. W., Pratt P. C., Crapo J. D. Characterization of three types of chrysotile asbestos after aerosolization. Environ Res. 1983 Jun;31(1):32–53. doi: 10.1016/0013-9351(83)90060-9. [DOI] [PubMed] [Google Scholar]
- Pinkerton K. E., Pratt P. C., Brody A. R., Crapo J. D. Fiber localization and its relationship to lung reaction in rats after chronic inhalation of chrysotile asbestos. Am J Pathol. 1984 Dec;117(3):484–498. [PMC free article] [PubMed] [Google Scholar]
- Roggli V. L., Brody A. R. Changes in numbers and dimensions of chrysotile asbestos fibers in lungs of rats following short-term exposure. Exp Lung Res. 1984;7(2):133–147. doi: 10.3109/01902148409069674. [DOI] [PubMed] [Google Scholar]
- Saint-Remy J. M., Cole P. Interactions of chrysotile asbestos fibres with the complement system. Immunology. 1980 Oct;41(2):431–437. [PMC free article] [PubMed] [Google Scholar]
- Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970 Jun;26(1):57–60. [PubMed] [Google Scholar]
- Schoenberger C. I., Hunninghake G. W., Kawanami O., Ferrans V. J., Crystal R. G. Role of alveolar macrophages in asbestosis: modulation of neutrophil migration to the lung after acute asbestos exposure. Thorax. 1982 Nov;37(11):803–809. doi: 10.1136/thx.37.11.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiep B. L., Carter R., Nicotra B., Berry J., Phillips R. E., Otsap B. Demand oxygen delivery during exercise. Chest. 1987 Jan;91(1):15–20. doi: 10.1378/chest.91.1.15. [DOI] [PubMed] [Google Scholar]
- Wagner J. C., Berry G., Skidmore J. W., Timbrell V. The effects of the inhalation of asbestos in rats. Br J Cancer. 1974 Mar;29(3):252–269. doi: 10.1038/bjc.1974.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. C. The sequelae of exposure to asbestos dust. Ann N Y Acad Sci. 1965 Dec 31;132(1):691–695. doi: 10.1111/j.1749-6632.1965.tb41147.x. [DOI] [PubMed] [Google Scholar]
- Warheit D. B., Chang L. Y., Hill L. H., Hook G. E., Crapo J. D., Brody A. R. Pulmonary macrophage accumulation and asbestos-induced lesions at sites of fiber deposition. Am Rev Respir Dis. 1984 Feb;129(2):301–310. [PubMed] [Google Scholar]
- Warheit D. B., George G., Hill L. H., Snyderman R., Brody A. R. Inhaled asbestos activates a complement-dependent chemoattractant for macrophages. Lab Invest. 1985 May;52(5):505–514. [PubMed] [Google Scholar]
- Warheit D. B., Hill L. H., George G., Brody A. R. Time course of chemotactic factor generation and the corresponding macrophage response to asbestos inhalation. Am Rev Respir Dis. 1986 Jul;134(1):128–133. doi: 10.1164/arrd.1986.134.1.128. [DOI] [PubMed] [Google Scholar]
- Wilson M. R., Gaumer H. R., Salvaggio J. E. Activation of the alternative complement pathway and generation of chemotactic factors by asbestos. J Allergy Clin Immunol. 1977 Oct;60(4):218–222. doi: 10.1016/0091-6749(77)90133-6. [DOI] [PubMed] [Google Scholar]