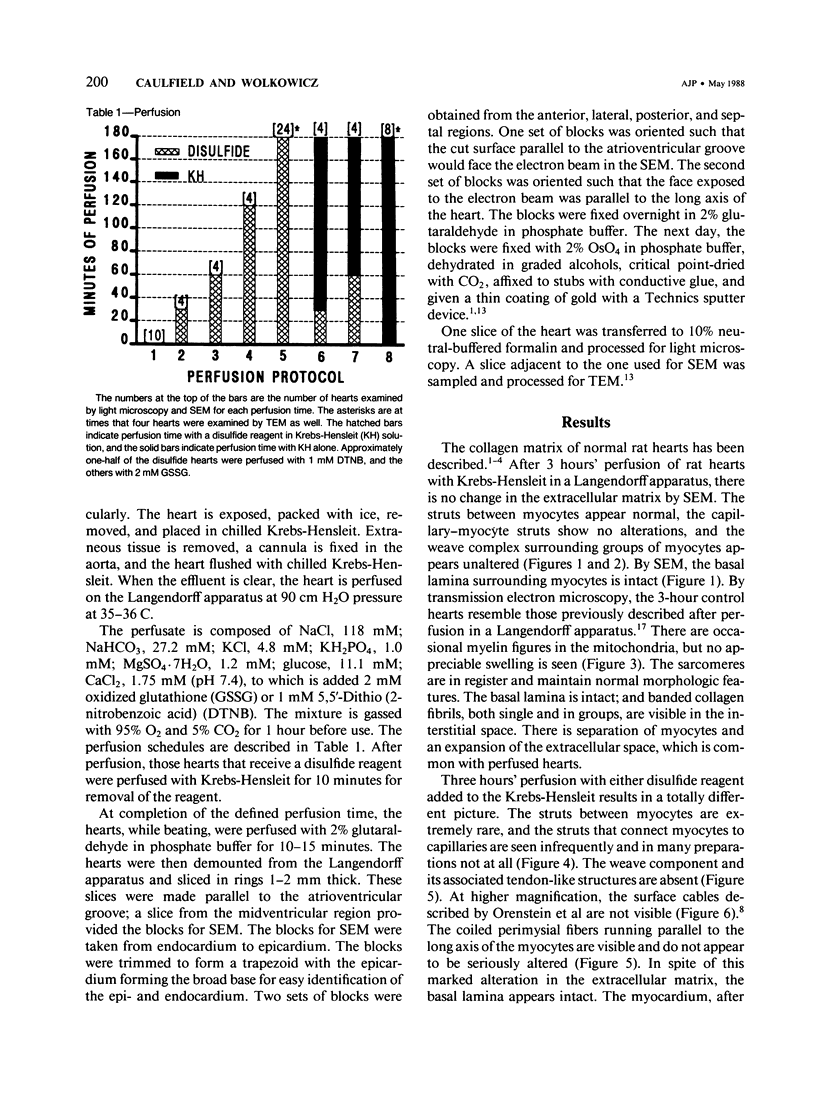
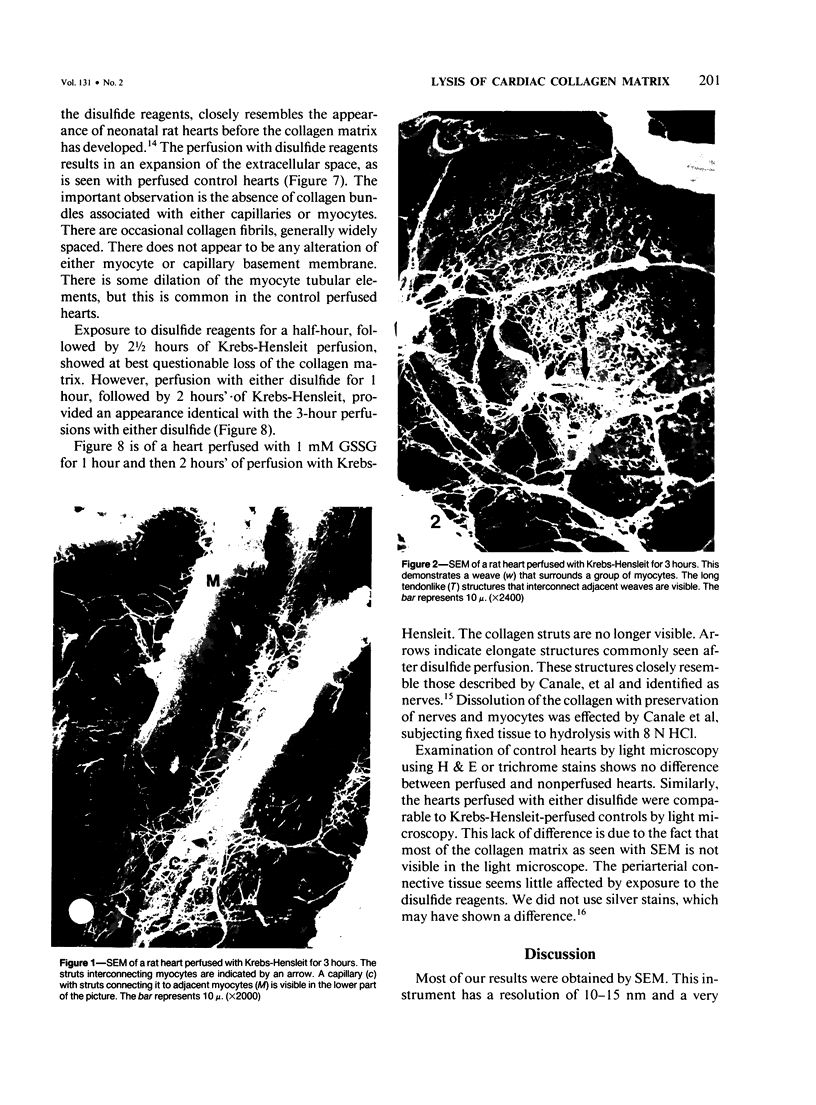
Abstract
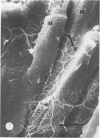
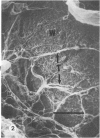
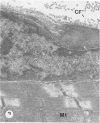
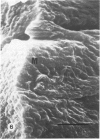
There is an extensive collagen network in the heart. The precise anatomy and function of this system has not been fully elucidated. The system does appear to contribute to diastolic compliance, and evidence indicates that the system may be important in directing the stress generated by sarcomeres to the ventricular cavity. Little is known about the mechanisms controlling collagen deposition and resorption in the heart. In this paper the authors demonstrate that disulfide reagents are capable of inducing a collagenolytic reaction in the isolated perfused heart that removes all components of the collagen matrix of the heart as visualized by scanning electron microscopy. The expression of collagenolytic activity requires perfusion of the heart for 1 hour with a disulfide reagent followed by 2 hours with Krebs-Hensleit alone. These results suggest that an inducible and active collagenolytic system exists in cardiac tissue and that this system may be expressed under conditions of oxidative stress.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arborgh B., Bell P., Brunk U., Collins V. P. The osmotic effect of glutaraldehyde during fixation. A transmission electron microscopy, scanning electron microscopy and cytochemical study. J Ultrastruct Res. 1976 Sep;56(3):339–350. doi: 10.1016/s0022-5320(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Borg T. K., Caulfield J. B. Collagen in the heart. Tex Rep Biol Med. 1979;39:321–333. [PubMed] [Google Scholar]
- Borg T. K., Caulfield J. B. The collagen matrix of the heart. Fed Proc. 1981 May 15;40(7):2037–2041. [PubMed] [Google Scholar]
- Borg T. K., Ranson W. F., Moslehy F. A., Caulfield J. B. Structural basis of ventricular stiffness. Lab Invest. 1981 Jan;44(1):49–54. [PubMed] [Google Scholar]
- Caulfield J. B., Borg T. K., Abel F. L. The effects of systole on left ventricular blood flow. Adv Myocardiol. 1983;4:379–393. doi: 10.1007/978-1-4757-4441-5_35. [DOI] [PubMed] [Google Scholar]
- Caulfield J. B., Borg T. K. The collagen network of the heart. Lab Invest. 1979 Mar;40(3):364–372. [PubMed] [Google Scholar]
- Cawston T. E., Murphy G. Mammalian collagenases. Methods Enzymol. 1981;80(Pt 100):711–722. doi: 10.1016/s0076-6879(81)80054-7. [DOI] [PubMed] [Google Scholar]
- Curello S., Ceconi C., Bigoli C., Ferrari R., Albertini A., Guarnieri C. Changes in the cardiac glutathione status after ischemia and reperfusion. Experientia. 1985 Jan 15;41(1):42–43. doi: 10.1007/BF02005863. [DOI] [PubMed] [Google Scholar]
- Gilbert H. F. Biological disulfides: the third messenger? Modulation of phosphofructokinase activity by thiol/disulfide exchange. J Biol Chem. 1982 Oct 25;257(20):12086–12091. [PubMed] [Google Scholar]
- Harper E., Bloch K. J., Gross J. The zymogen of tadpole collagenase. Biochemistry. 1971 Aug 3;10(16):3035–3041. doi: 10.1021/bi00792a008. [DOI] [PubMed] [Google Scholar]
- Orenstein J., Hogan D., Bloom S. Surface cables of cardiac myocytes. J Mol Cell Cardiol. 1980 Aug;12(8):771–780. doi: 10.1016/0022-2828(80)90079-6. [DOI] [PubMed] [Google Scholar]
- Robinson T. F., Cohen-Gould L., Factor S. M. Skeletal framework of mammalian heart muscle. Arrangement of inter- and pericellular connective tissue structures. Lab Invest. 1983 Oct;49(4):482–498. [PubMed] [Google Scholar]
- Robinson T. F., Cohen-Gould L., Remily R. M., Capasso J. M., Factor S. M. Extracellular structures in heart muscle. Adv Myocardiol. 1985;5:243–255. doi: 10.1007/978-1-4757-1287-2_19. [DOI] [PubMed] [Google Scholar]
- Robinson T. F., Winegrad S. A variety of intercellular connections in heart muscle. J Mol Cell Cardiol. 1981 Feb;13(2):185–195. doi: 10.1016/0022-2828(81)90215-7. [DOI] [PubMed] [Google Scholar]
- Sato S., Ashraf M., Millard R. W., Fujiwara H., Schwartz A. Connective tissue changes in early ischemia of porcine myocardium: an ultrastructural study. J Mol Cell Cardiol. 1983 Apr;15(4):261–275. doi: 10.1016/0022-2828(83)90281-x. [DOI] [PubMed] [Google Scholar]
- Tschesche H., Macartney H. W. A new principle of regulation of enzymic activity. Activation and regulation of human polymorphonuclear leukocyte collagenase via disulfide-thiol exchange as catalysed by the glutathione cycle in a peroxidase-coupled reaction to glucose metabolism. Eur J Biochem. 1981 Nov;120(1):183–190. doi: 10.1111/j.1432-1033.1981.tb05687.x. [DOI] [PubMed] [Google Scholar]
- Weissler A. M., Kruger F. A., Baba N., Scarpelli D. G., Leighton R. F., Gallimore J. K. Role of anaerobic metabolism in the preservation of functional capacity and structure of anoxic myocardium. J Clin Invest. 1968 Feb;47(2):403–416. doi: 10.1172/JCI105737. [DOI] [PMC free article] [PubMed] [Google Scholar]