Abstract
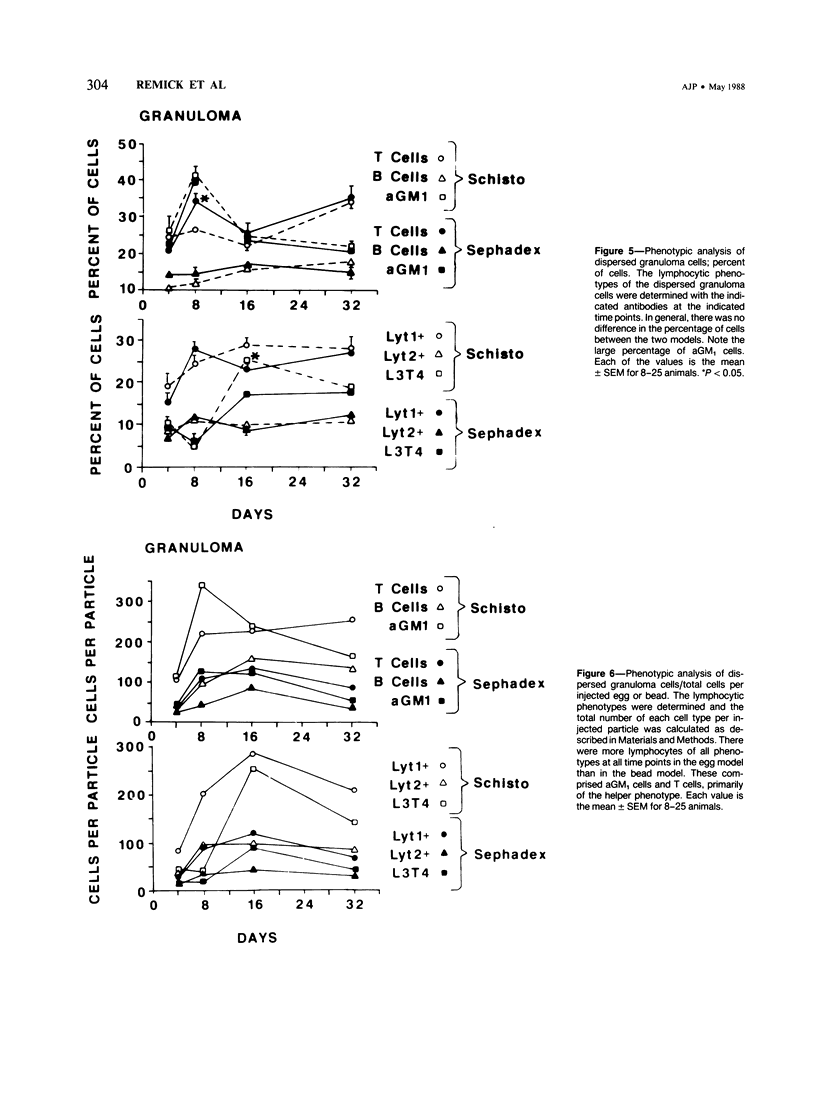
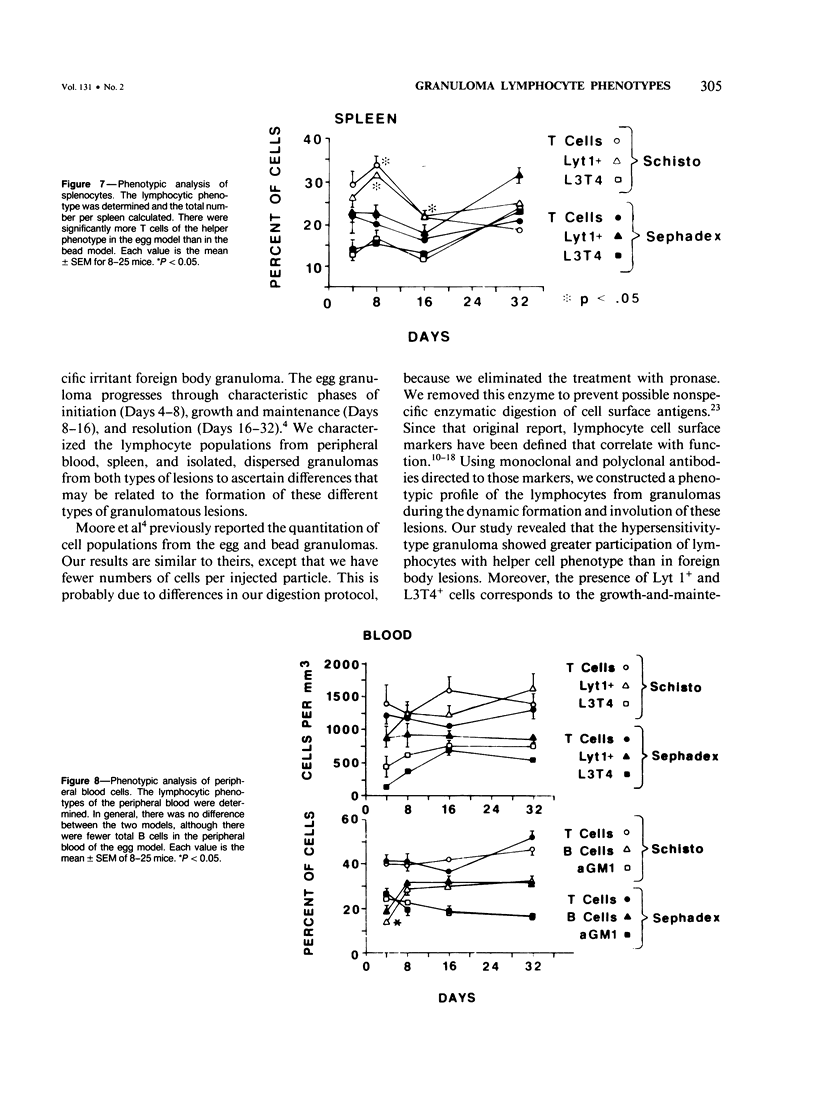
Synchronous models of T-cell-mediated and foreign body granulomas were induced in mice by intravenous embolization of Schistosoma mansoni eggs and Sephadex beads, respectively. The authors then performed flow-cytometric analysis of lymphocytes from dispersed granulomas, spleens, and peripheral blood at 4, 8, 16, and 32 days corresponding to the induction, growth, and maintenance, and resolution of these lesions. Lymphocytes were identified on the basis of light scatter characteristics, and the nature of the cells was confirmed by cell sorting and electron-microscopic examination. Lymphocyte subpopulations were characterized with antibodies to lymphocyte surface markers, specifically Ig, Thy 1.2, Lyt 1, Lyt 2, and L3T4. Natural killer cells were identified with anti-asialo GM1. Egg-induced granulomas had more lymphocytes of all phenotypes at all time points. Surprisingly, there was a significant number of cells staining positive for asialo GM1. On Day 16 after embolization there was a greater percentage of helper T cells, as defined by positive staining with L3T4, in the egg model, compared with the bead model. There was no obvious shift of lymphocytes from either the blood or spleen into the granuloma. These data confirm the importance of T cells in the direct participation of granulomatous inflammation, and the large numbers of asialo GM1-positive cells suggest a role for natural killer cells.
Full text
PDF


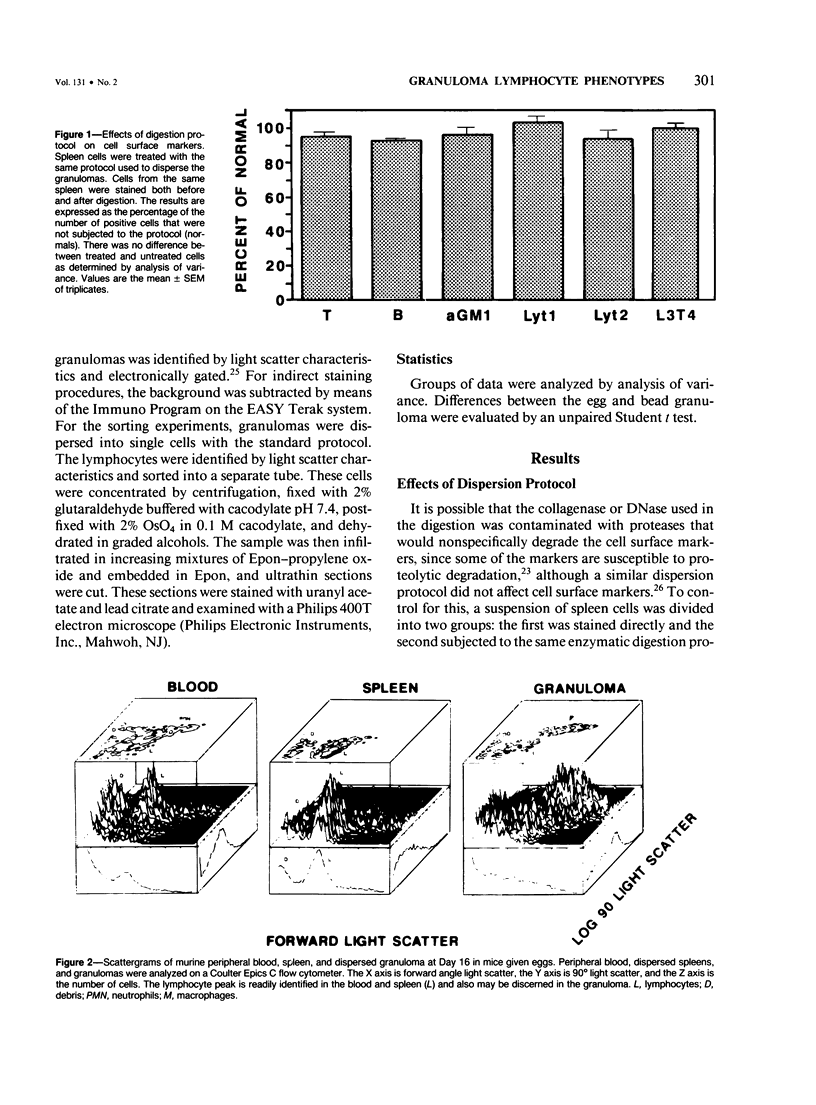
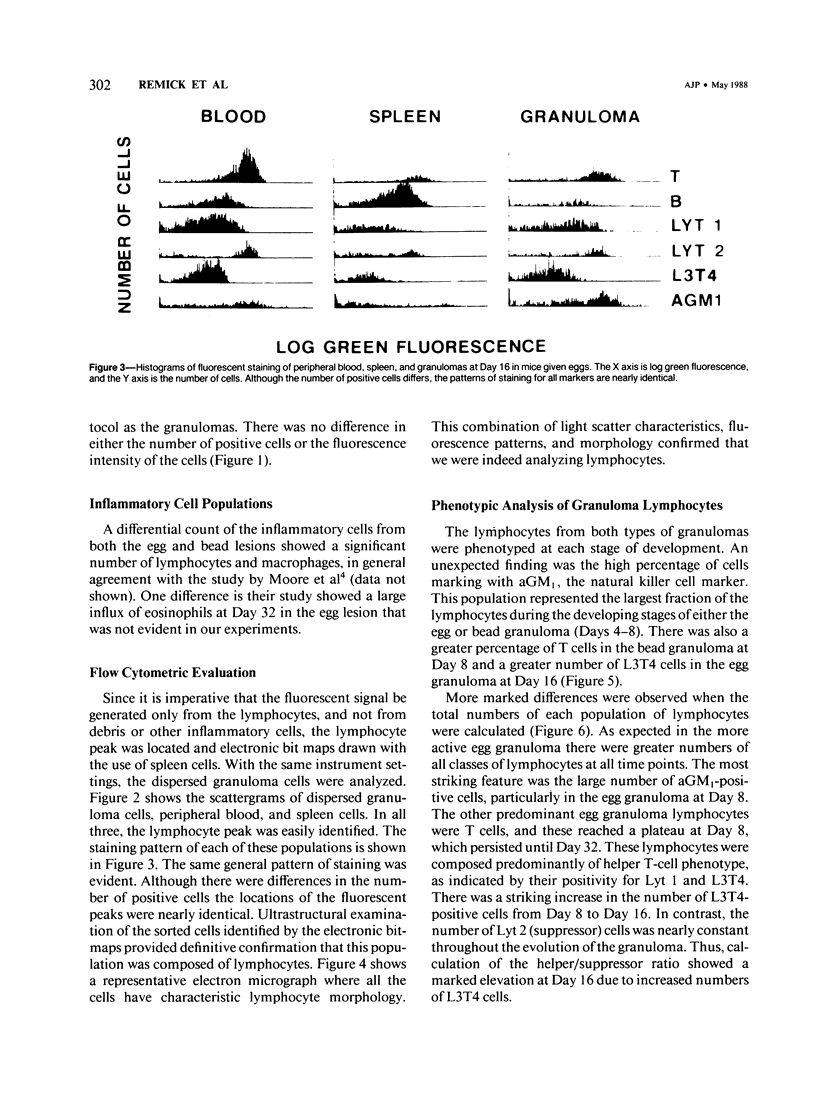


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COKER C. M., LICHTENBERG F. A revised method for isolation of Schistosoma mansoni eggs for biological experimentation. Proc Soc Exp Biol Med. 1956 Aug-Sep;92(4):780–782. doi: 10.3181/00379727-92-22612. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T lymphocytes bearing different Ly antigens. II. Cooperation between subclasses of Ly+ cells in the generation of killer activity. J Exp Med. 1975 Jun 1;141(6):1390–1399. doi: 10.1084/jem.141.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Shen F. W., Boyse E. A. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1391–1340. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L., David C. S. Regulation of granulomatous inflammation in murine schistosomiasis. In vitro characterization of T lymphocyte subsets involved in the production and suppression of migration inhibition factor. J Exp Med. 1980 Jun 1;151(6):1398–1412. doi: 10.1084/jem.151.6.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L. Modulation of granulomatous hypersensitivity. I. Characterization of T lymphocytes involved in the adoptive suppression of granuloma formation in Schistosoma mansoni-infected mice. J Immunol. 1979 Sep;123(3):1409–1414. [PubMed] [Google Scholar]
- Chensue S. W., Wellhausen S. R., Boros D. L. Modulation of granulomatous hypersensitivity. II. Participation of Ly 1+ and Ly 2+ T lymphocytes in the suppression of granuloma formation and lymphokine production in Schistosoma mansoni-infected mice. J Immunol. 1981 Jul;127(1):363–367. [PubMed] [Google Scholar]
- Colley D. G. Adoptive suppression of granuloma formation. J Exp Med. 1976 Mar 1;143(3):696–700. doi: 10.1084/jem.143.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Domingo E. O., Warren K. S. The inhibition of granuloma formation around Schistosoma mansoni eggs. II. Thymectomy. Am J Pathol. 1967 Nov;51(5):757–767. [PMC free article] [PubMed] [Google Scholar]
- Geboes K., van den Oord J., De Wolf-Peeters C., Desmet V., Rutgeerts P., Janssens J., Vantrappen G., Penninckx F., Kerremans R. The cellular composition of granulomas in mesenteric lymph nodes from patients with Crohn's disease. Virchows Arch A Pathol Anat Histopathol. 1986;409(5):679–692. doi: 10.1007/BF00713433. [DOI] [PubMed] [Google Scholar]
- Gupta S. K., Bhutani L. K., Nath I. The in situ characteristics of mononuclear cell infiltrates in dermal lesions of leprosy. Int J Lepr Other Mycobact Dis. 1982 Sep;50(3):297–305. [PubMed] [Google Scholar]
- Habu S., Hayakawa K., Okumura K., Tada T. Surface markers on natural killer cells of the mouse. Eur J Immunol. 1979 Dec;9(12):938–942. doi: 10.1002/eji.1830091206. [DOI] [PubMed] [Google Scholar]
- Holt P. G., Robinson B. W., Reid M., Kees U. R., Warton A., Dawson V. H., Rose A., Schon-Hegrad M., Papadimitriou J. M. Extraction of immune and inflammatory cells from human lung parenchyma: evaluation of an enzymatic digestion procedure. Clin Exp Immunol. 1986 Oct;66(1):188–200. [PMC free article] [PubMed] [Google Scholar]
- Huber B., Devinsky O., Gershon R. K., Cantor H. Cell-mediated immunity: delayed-type hypersensitivity and cytotoxic responses are mediated by different T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1534–1539. doi: 10.1084/jem.143.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Crystal R. G. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981 Aug 20;305(8):429–434. doi: 10.1056/NEJM198108203050804. [DOI] [PubMed] [Google Scholar]
- Kasai M., Iwamori M., Nagai Y., Okumura K., Tada T. A glycolipid on the surface of mouse natural killer cells. Eur J Immunol. 1980 Mar;10(3):175–180. doi: 10.1002/eji.1830100304. [DOI] [PubMed] [Google Scholar]
- Lammie P. J., Linette G. P., Phillips S. M. Characterization of Schistosoma mansoni antigen-reactive T cell clones that form granulomas in vitro. J Immunol. 1985 Jun;134(6):4170–4175. [PubMed] [Google Scholar]
- Lanier L. L., Warner N. L. Paraformaldehyde fixation of hematopoietic cells for quantitative flow cytometry (FACS) analysis. J Immunol Methods. 1981;47(1):25–30. doi: 10.1016/0022-1759(81)90253-2. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980 Aug 1;152(2):280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Seaman W. E., Tsu T. T., Herzenberg L. A. Lyt-2 and lyt-3 antigens are on two different polypeptide subunits linked by disulfide bonds. Relationship of subunits to T cell cytolytic activity. J Exp Med. 1981 Jun 1;153(6):1503–1516. doi: 10.1084/jem.153.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Seaman W. E., Tsu T. T., Herzenberg L. A. Lyt-2 and lyt-3 antigens are on two different polypeptide subunits linked by disulfide bonds. Relationship of subunits to T cell cytolytic activity. J Exp Med. 1981 Jun 1;153(6):1503–1516. doi: 10.1084/jem.153.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley J., Haregewoin A., Yemaneberhan T., Warndorff van Diepen T., Nsibami J., Knowles D., Smith K. A., Godal T. In vivo responses to Mycobacterium leprae: antigen presentation, interleukin-2 production, and immune cell phenotypes in naturally occurring leprosy lesions. Int J Lepr Other Mycobact Dis. 1985 Sep;53(3):385–394. [PubMed] [Google Scholar]
- Lovett E. J., 3rd, Schnitzer B., Keren D. F., Flint A., Hudson J. L., McClatchey K. D. Application of flow cytometry to diagnostic pathology. Lab Invest. 1984 Feb;50(2):115–140. [PubMed] [Google Scholar]
- Modlin R. L., Hofman F. M., Meyer P. R., Sharma O. P., Taylor C. R., Rea T. H. In situ demonstration of T lymphocyte subsets in granulomatous inflammation: leprosy, rhinoscleroma and sarcoidosis. Clin Exp Immunol. 1983 Mar;51(3):430–438. [PMC free article] [PubMed] [Google Scholar]
- Modlin R. L., Horwitz D. A., Jordan R. R., Gebhard J. F., Taylor C. R., Rea T. H. Immunopathologic demonstration of T lymphocyte subpopulations and interleukin 2 in granuloma annulare. Pediatr Dermatol. 1984 Jul;2(1):26–32. doi: 10.1111/j.1525-1470.1984.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Moore D. L., Grove D. I., Warren K. S. The Schistosoma mansoni egg granuloma: quantitation of cell populations. J Pathol. 1977 Jan;121(1):41–50. doi: 10.1002/path.1711210107. [DOI] [PubMed] [Google Scholar]
- Myrvik Q. N. Immunopathology of pulmonary granulomas. Adv Exp Med Biol. 1982;155:649–657. doi: 10.1007/978-1-4684-4394-3_71. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Kaplan G., Sarno E. N., Horwitz M. A., Steinman R. M., Levis W. R., Nogueira N., Hair L. S., Gattass C. R., Arrick B. A. The cutaneous infiltrates of leprosy: cellular characteristics and the predominant T-cell phenotypes. N Engl J Med. 1982 Dec 23;307(26):1593–1597. doi: 10.1056/NEJM198212233072601. [DOI] [PubMed] [Google Scholar]
- Warren K. S. A functional classification of granulomatous inflammation. Ann N Y Acad Sci. 1976;278:7–18. doi: 10.1111/j.1749-6632.1976.tb47011.x. [DOI] [PubMed] [Google Scholar]
- Warren K. S., Domingo E. O., Cowan R. B. Granuloma formation around schistosome eggs as a manifestation of delayed hypersensitivity. Am J Pathol. 1967 Nov;51(5):735–756. [PMC free article] [PubMed] [Google Scholar]