Abstract
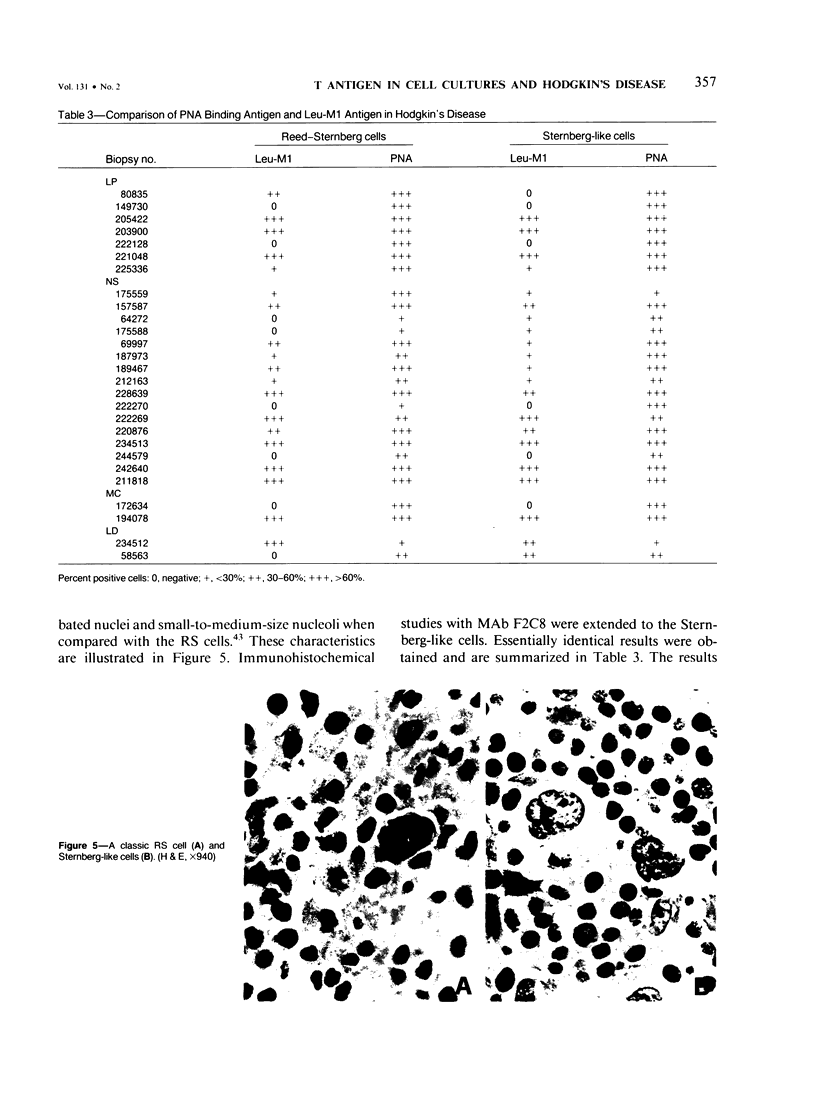
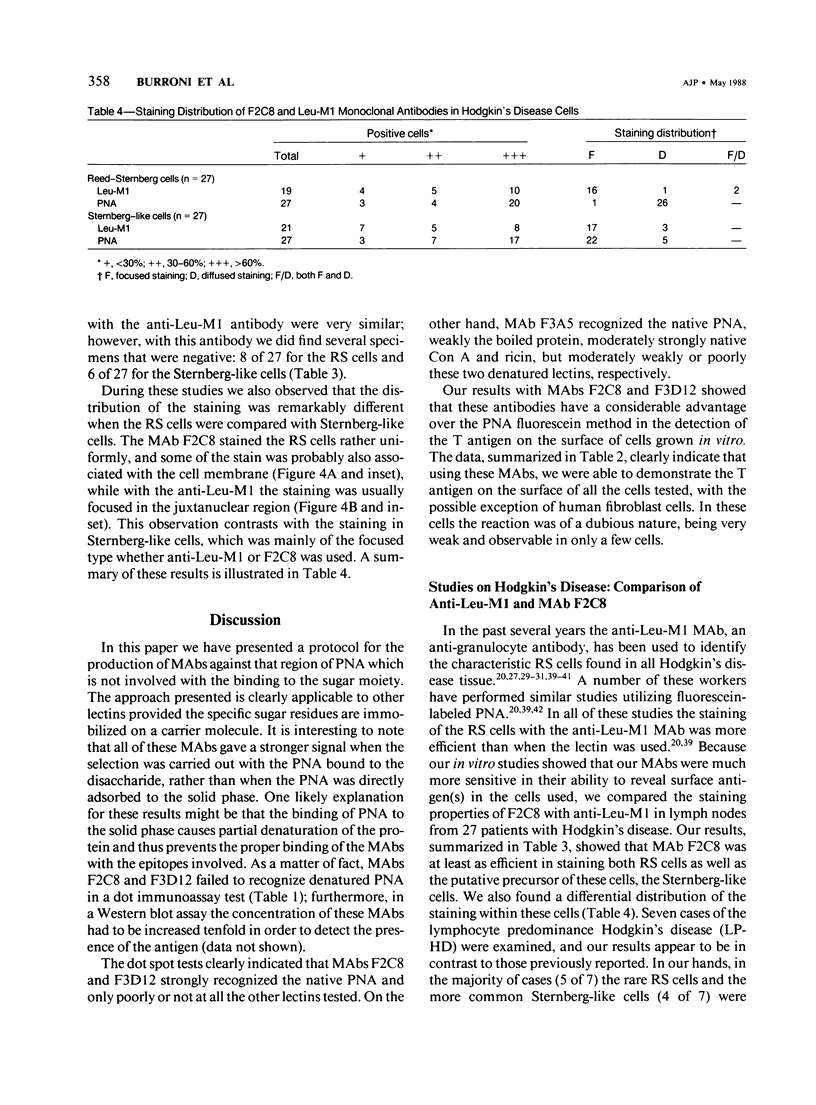
The purpose of this study was to increase the sensitivity of the staining reaction for the T antigen on the surface of neoplastic cells grown in vitro with the use of site-specific monoclonal antibodies (MAbs). The authors describe anti-peanut agglutinin (PNA) MAbs selected by screening the hybridomas with PNA and PNA bound to bovine serum albumin conjugated with the T antigen. The selected hybridomas (F2C8, F3D12, F3A5) were then grown in pristane-sensitized mice or in the Amicon Hollow Fiber System (F2C8). The affinity constant values for PNA were measured, and all the purified MAbs were tested on both native and denatured PNA, wheat germ agglutinin, concanavalin A, and ricin by using the immunoassay dot test and immunoblotting methods. Eleven different cell lines were stained with the three MAbs; similar results were obtained with F2C8 and F3D12. In each case the fluorescence, if present, was associated with the cell membrane, and the intensity of the staining was always stronger when the cells were incubated with the MAbs than when stained with fluorescein-labeled PNA. On the other hand, F3A5 failed to stain unfixed cells preincubated with PNA but stained the same cells after fixation, independently of the presence of PNA. One of the antibodies, F2C8, was used to stain histologic preparations from 27 cases of Hodgkin's disease and was compared with the anti-granulocyte antibody, Leu-M1, which has been used by numerous authors to identify the characteristic Reed-Sternberg cells. The results obtained were qualitatively similar; ie, F2C8 was at least as efficient as anti-Leu-M1 in its ability to stain the typical diagnostic cells in Hodgkin's disease.
Full text
PDF

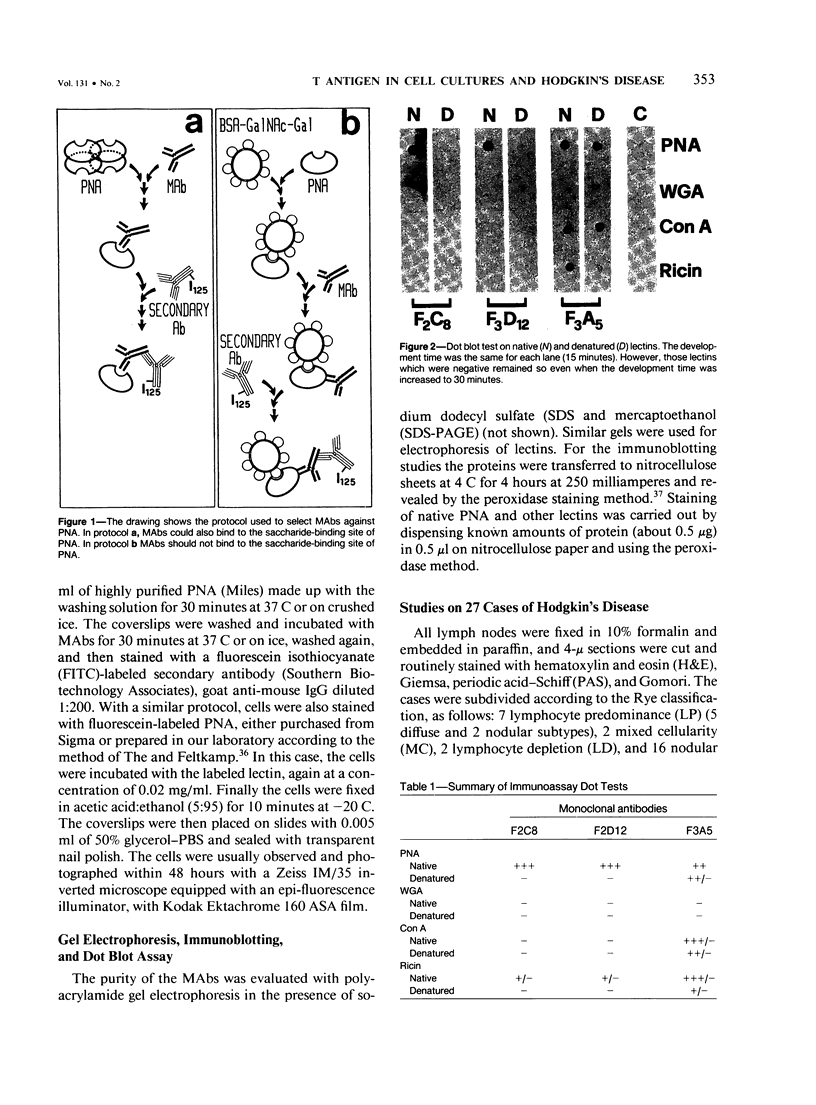
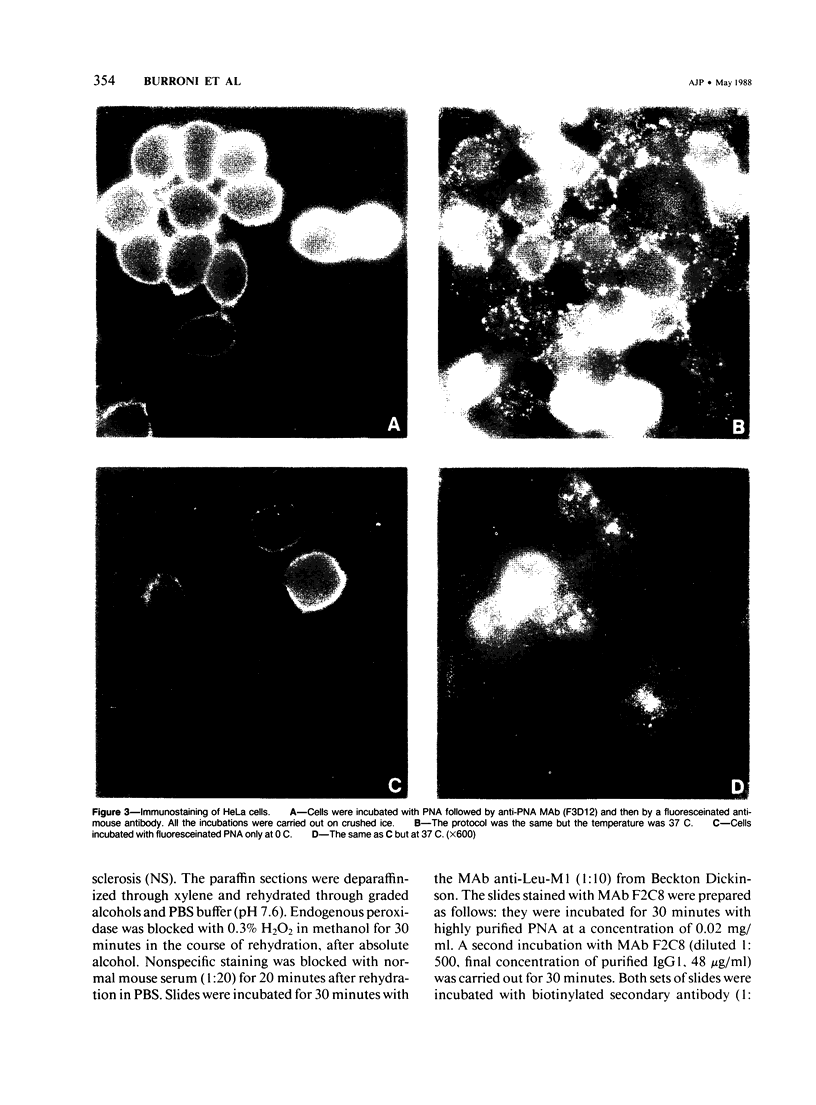




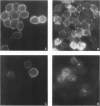
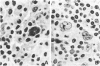
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoni G., Mariani M. An interactive computer program for the determination of the binding constants of monoclonal antibodies by non-linear regression analysis of radioimmunoassay data. J Immunol Methods. 1985 Oct 24;83(1):61–68. doi: 10.1016/0022-1759(85)90058-4. [DOI] [PubMed] [Google Scholar]
- Bychkov V., Toto P. D. Lectin binding to normal, dysplastic, and neoplastic cervical epithelium. Am J Clin Pathol. 1986 May;85(5):542–547. doi: 10.1093/ajcp/85.5.542. [DOI] [PubMed] [Google Scholar]
- Cianfriglia M., Armellini D., Massone A., Mariani M. Simple immunization protocol for high frequency production of soluble antigen-specific hybridomas. Hybridoma. 1983;2(4):451–457. doi: 10.1089/hyb.1983.2.451. [DOI] [PubMed] [Google Scholar]
- De Maio A., Lis H., Gershoni J. M., Sharon N. Identification of glycoproteins that are receptors for peanut agglutinin on immature (cortical) mouse thymocytes. FEBS Lett. 1986 Jan 1;194(1):28–32. doi: 10.1016/0014-5793(86)80045-x. [DOI] [PubMed] [Google Scholar]
- De Maio A., Lis H., Gershoni J. M., Sharon N. Identification of peanut agglutinin-binding glycoproteins on immature human thymocytes. Cell Immunol. 1986 May;99(2):345–353. doi: 10.1016/0008-8749(86)90243-1. [DOI] [PubMed] [Google Scholar]
- Dorfman R. F., Gatter K. C., Pulford K. A., Mason D. Y. An evaluation of the utility of anti-granulocyte and anti-leukocyte monoclonal antibodies in the diagnosis of Hodgkin's disease. Am J Pathol. 1986 Jun;123(3):508–519. [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Favero J., Bonnafous J. C., Dornand J., Mani J. C. Characterization of peanut agglutinin receptors of murine thymocytes. Cell Immunol. 1984 Jul;86(2):439–447. doi: 10.1016/0008-8749(84)90399-x. [DOI] [PubMed] [Google Scholar]
- Hogeman P. H., Veerman A. J., Van Zantwijk C. H., Huismans D. R. Peanut agglutinin distinguishes CALLA positive reactive bone marrow cells from leukaemic blasts. Br J Haematol. 1985 Feb;59(2):392–394. doi: 10.1111/j.1365-2141.1985.tb03005.x. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Ho Y. S., Li P. J., Monheit J., Ree H. J., Sheibani K., Winberg C. D. L&H variants of Reed-Sternberg cells express sialylated Leu M1 antigen. Am J Pathol. 1986 Feb;122(2):199–203. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Huang L. C., Hsu P. L., Ge Z. H., Ho Y. S., Cuttita F., Mulshine J. Biochemical and ultrastructural study of Leu M1 antigen in Reed-Sternberg cells: comparison with granulocytes and interdigitating reticulum cells. J Natl Cancer Inst. 1986 Aug;77(2):363–370. [PubMed] [Google Scholar]
- Hsu S. M., Jaffe E. S. Leu M1 and peanut agglutinin stain the neoplastic cells of Hodgkin's disease. Am J Clin Pathol. 1984 Jul;82(1):29–32. doi: 10.1093/ajcp/82.1.29. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Yang K., Jaffe E. S. Phenotypic expression of Hodgkin's and Reed-Sternberg cells in Hodgkin's disease. Am J Pathol. 1985 Feb;118(2):209–217. [PMC free article] [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Momoi T., Momoi M. Y., Kurata T. Peanut agglutinin receptor is a marker of myelin in rat brain. Developmental changes in its distribution. J Neurochem. 1986 Jan;46(1):229–234. doi: 10.1111/j.1471-4159.1986.tb12951.x. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. The interactions of lectins with animal cell surfaces. Int Rev Cytol. 1974;39:89–190. doi: 10.1016/s0074-7696(08)60939-0. [DOI] [PubMed] [Google Scholar]
- Orntoft T. F., Mors N. P., Eriksen G., Jacobsen N. O., Poulsen H. S. Comparative immunoperoxidase demonstration of T-antigens in human colorectal carcinomas and morphologically abnormal mucosa. Cancer Res. 1985 Jan;45(1):447–452. [PubMed] [Google Scholar]
- Pinkus G. S., Said J. W. Hodgkin's disease, lymphocyte predominance type, nodular--a distinct entity? Unique staining profile for L&H variants of Reed-Sternberg cells defined by monoclonal antibodies to leukocyte common antigen, granulocyte-specific antigen, and B-cell-specific antigen. Am J Pathol. 1985 Jan;118(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Pinkus G. S., Thomas P., Said J. W. Leu-M1--a marker for Reed-Sternberg cells in Hodgkin's disease. An immunoperoxidase study of paraffin-embedded tissues. Am J Pathol. 1985 May;119(2):244–252. [PMC free article] [PubMed] [Google Scholar]
- Poppema S., Kaiserling E., Lennert K. Hodgkin's disease with lymphocytic predominance, nodular type (nodular paragranuloma) and progressively transformed germinal centres--a cytohistological study. Histopathology. 1979 Jul;3(4):295–308. doi: 10.1111/j.1365-2559.1979.tb03011.x. [DOI] [PubMed] [Google Scholar]
- Ree H. J. Concanavalin A-binding histiocytes in Hodgkin's disease. A predictor of early relapse. Cancer. 1986 Jul 1;58(1):87–95. doi: 10.1002/1097-0142(19860701)58:1<87::aid-cncr2820580116>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Ree H. J., Kadin M. E. Macrophage-histiocytes in Hodgkin's disease. The relation of peanut-agglutinin-binding macrophage-histiocytes to clinicopathologic presentation and course of disease. Cancer. 1985 Jul 15;56(2):333–338. doi: 10.1002/1097-0142(19850715)56:2<333::aid-cncr2820560222>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Ree H. J., Kadin M. E. Peanut agglutinin. A useful marker for histiocytosis X and interdigitating reticulum cells. Cancer. 1986 Jan 15;57(2):282–287. doi: 10.1002/1097-0142(19860115)57:2<282::aid-cncr2820570216>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Reisner Y., Gachelin G., Dubois P., Nicolas J. F., Sharon N., Jacob F. Interaction of peanut agglutinin, a lectin specific for nonreducing terminal D-galactosyl residues, with embryonal carcinoma cells. Dev Biol. 1977 Nov;61(1):20–27. doi: 10.1016/0012-1606(77)90338-4. [DOI] [PubMed] [Google Scholar]
- Reisner Y., Sharon N. Fractionation of subpopulations of mouse and human lymphocytes by peanut agglutinin or soybean agglutinin. Methods Enzymol. 1984;108:168–179. doi: 10.1016/s0076-6879(84)08084-8. [DOI] [PubMed] [Google Scholar]
- Schauenstein K., Globerson A., Rosenberg M., Sharon N., Wick G. Characterization of chicken lymphocyte subsets separated by peanut agglutinin. Cell Immunol. 1983 Sep;80(2):288–300. doi: 10.1016/0008-8749(83)90117-x. [DOI] [PubMed] [Google Scholar]
- Seitz R. C., Fischer K., Stegner H. E., Poschmann A. Detection of metastatic breast carcinoma cells by immunofluorescent demonstration of Thomsen-Friedenreich antigen. Cancer. 1984 Sep 1;54(5):830–836. doi: 10.1002/1097-0142(19840901)54:5<830::aid-cncr2820540512>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Sharon N. Carbohydrates as recognition determinants in phagocytosis and in lectin-mediated killing of target cells. Biol Cell. 1984;51(2):239–245. doi: 10.1111/j.1768-322x.1984.tb00304.x. [DOI] [PubMed] [Google Scholar]
- Sheibani K., Battifora H., Burke J. S., Rappaport H. Leu-M1 antigen in human neoplasms. An immunohistologic study of 400 cases. Am J Surg Pathol. 1986 Apr;10(4):227–236. doi: 10.1097/00000478-198604000-00001. [DOI] [PubMed] [Google Scholar]
- Skutelsky E., Lotan R., Sharon N., Danon D. Distribution of the T-antigen on erythroid cell surfaces. Studies with peanut agglutinin, an anti-T specific lectin. Biochim Biophys Acta. 1977 Jun 2;467(2):165–174. doi: 10.1016/0005-2736(77)90193-6. [DOI] [PubMed] [Google Scholar]
- Springer G. F., Desai P. R. Extent of desialation of blood group MM, NN, and MN antigens required for reactivity with human anti-T antibody and Arachis hypogaea lectin. J Biol Chem. 1982 Mar 25;257(6):2744–2746. [PubMed] [Google Scholar]
- Springer G. F., Murthy S. M., Desai P. R., Fry W. A., Tegtmeyer H., Scanlon E. F. Patients' immune response to breast and lung carcinoma-associated Thomsen-Friedenreich (T) specificity. Klin Wochenschr. 1982 Feb 1;60(3):121–131. doi: 10.1007/BF01711276. [DOI] [PubMed] [Google Scholar]
- Springer G. F. T and Tn, general carcinoma autoantigens. Science. 1984 Jun 15;224(4654):1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- Strauchen J. A., Dimitriu-Bona A. Immunopathology of Hodgkin's disease. Characterization of Reed-Sternberg cells with monoclonal antibodies. Am J Pathol. 1986 May;123(2):293–300. [PMC free article] [PubMed] [Google Scholar]
- Strauchen J. A. Lectin receptors as markers of lymphoid cells. II. Reed-Sternberg cells share lectin-binding properties of monocyte macrophages. Am J Pathol. 1984 Sep;116(3):370–376. [PMC free article] [PubMed] [Google Scholar]
- The T. H., Feltkamp T. E. Conjugation of fluorescein isothiocyanate to antibodies. II. A reproducible method. Immunology. 1970 Jun;18(6):875–881. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerman A. J., Hogeman P. H., Huismans D. R., Van Zantwijk C. H., Bezemer P. D. Peanut agglutinin, a marker for T-cell acute lymphoblastic leukemia with a good prognosis. Cancer Res. 1985 Apr;45(4):1890–1893. [PubMed] [Google Scholar]
- White F. V., Ceccarini C., Georgieff I., Matthieu J. M., Costantino-Ceccarini E. Growth properties and biochemical characterization of mouse Schwann cells cultured in vitro. Exp Cell Res. 1983 Oct;148(1):183–194. doi: 10.1016/0014-4827(83)90198-2. [DOI] [PubMed] [Google Scholar]
- Wolf M. F., Koerner U., Schumacher K. Specificity of reagents directed to the Thomsen-Friedenreich antigen and their capacity to bind to the surface of human carcinoma cell lines. Cancer Res. 1986 Apr;46(4 Pt 1):1779–1782. [PubMed] [Google Scholar]
- Yuan M., Itzkowitz S. H., Boland C. R., Kim Y. D., Tomita J. T., Palekar A., Bennington J. L., Trump B. F., Kim Y. S. Comparison of T-antigen expression in normal, premalignant, and malignant human colonic tissue using lectin and antibody immunohistochemistry. Cancer Res. 1986 Sep;46(9):4841–4847. [PubMed] [Google Scholar]