Abstract
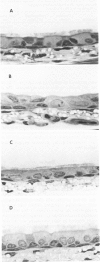
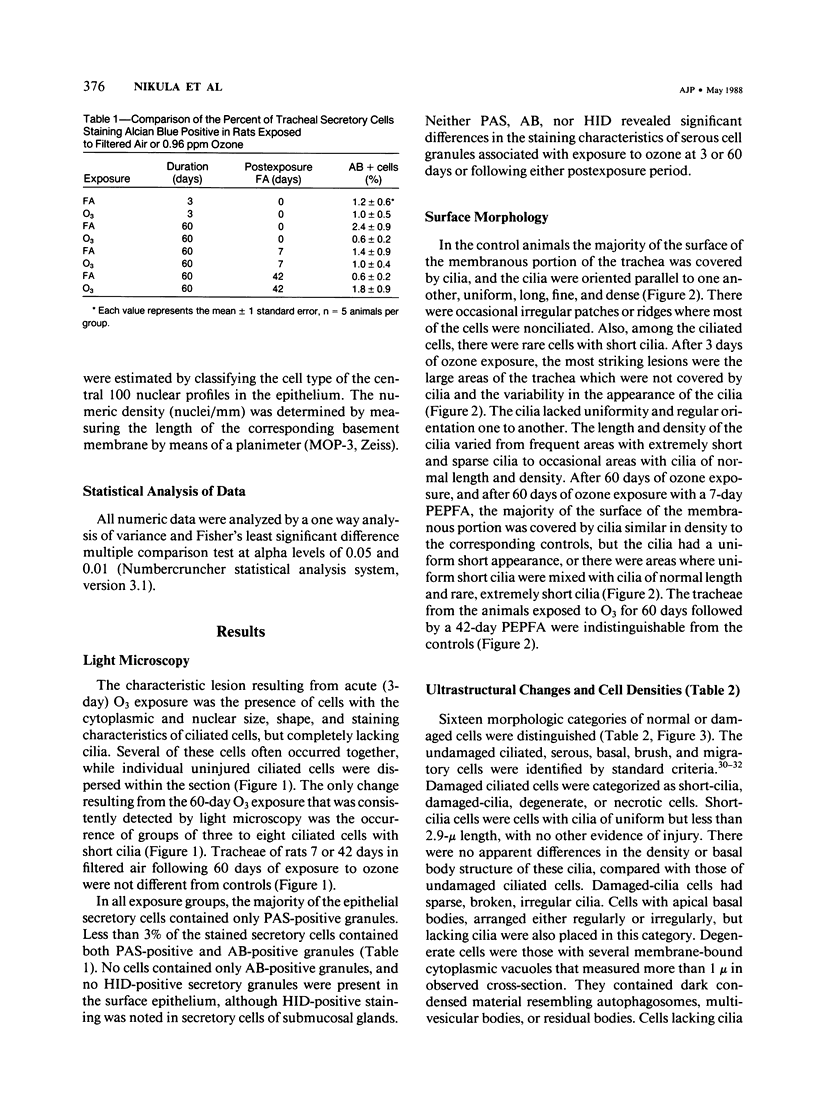
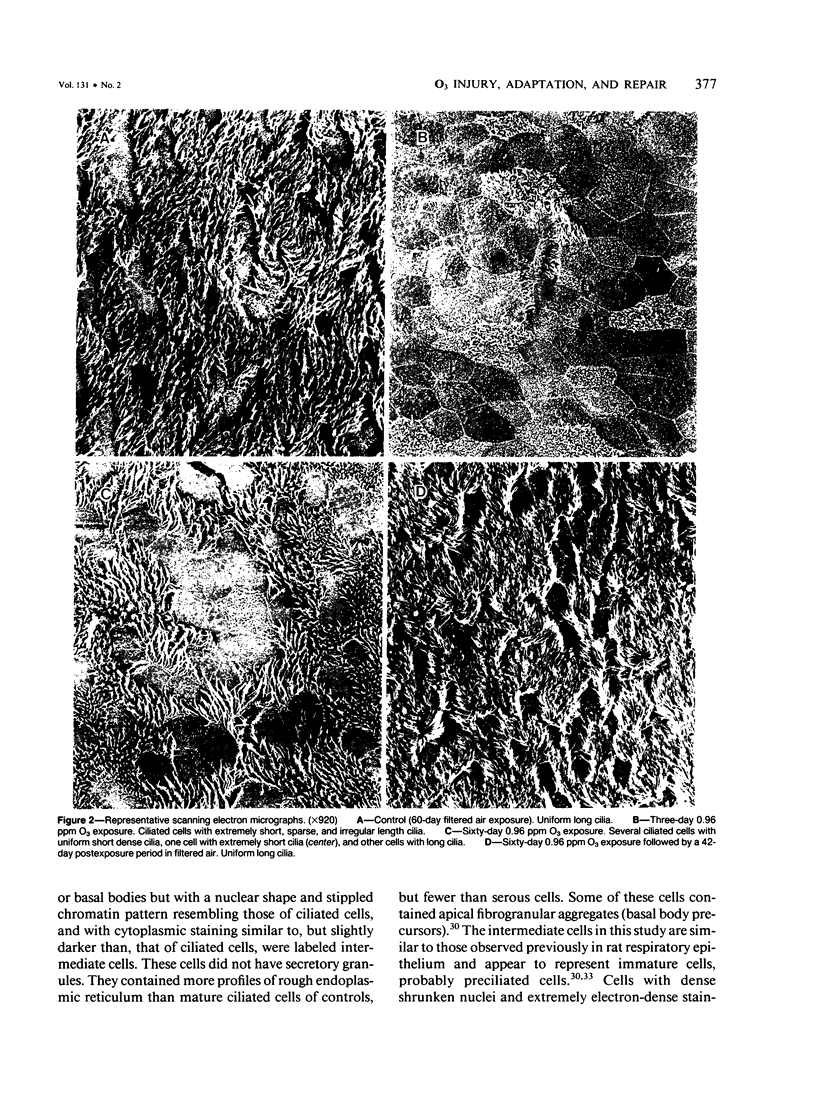
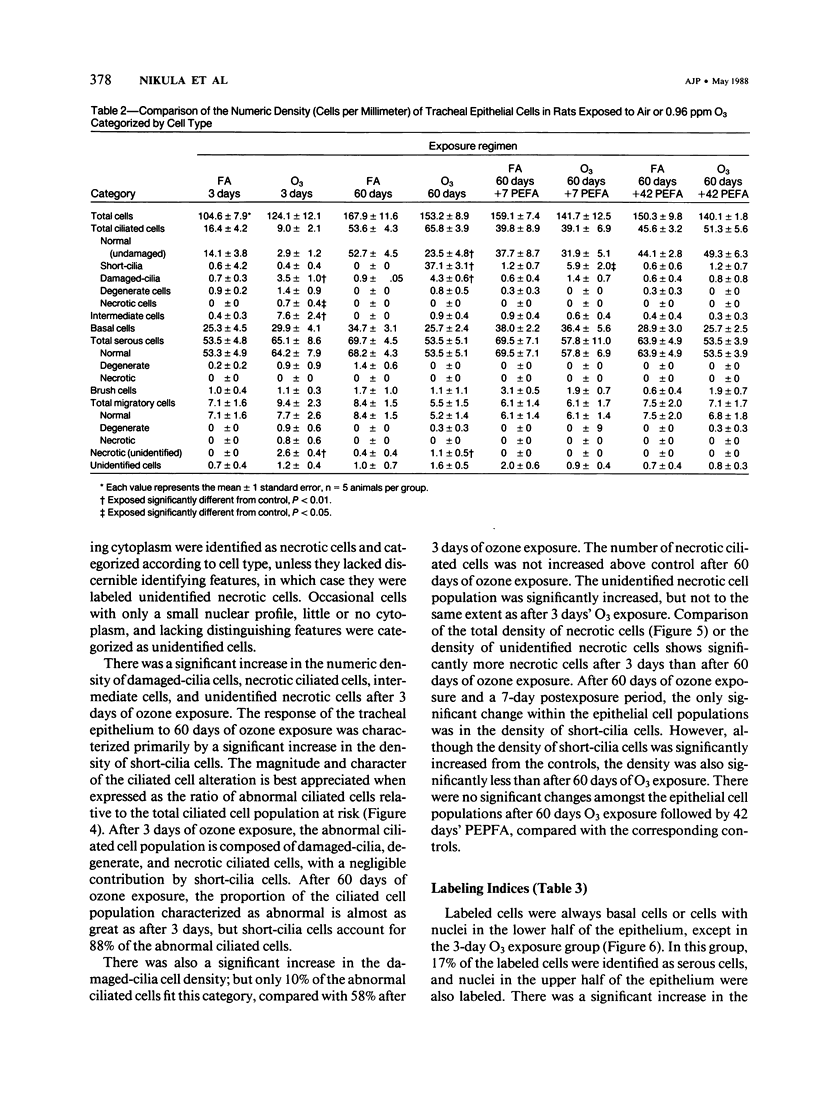
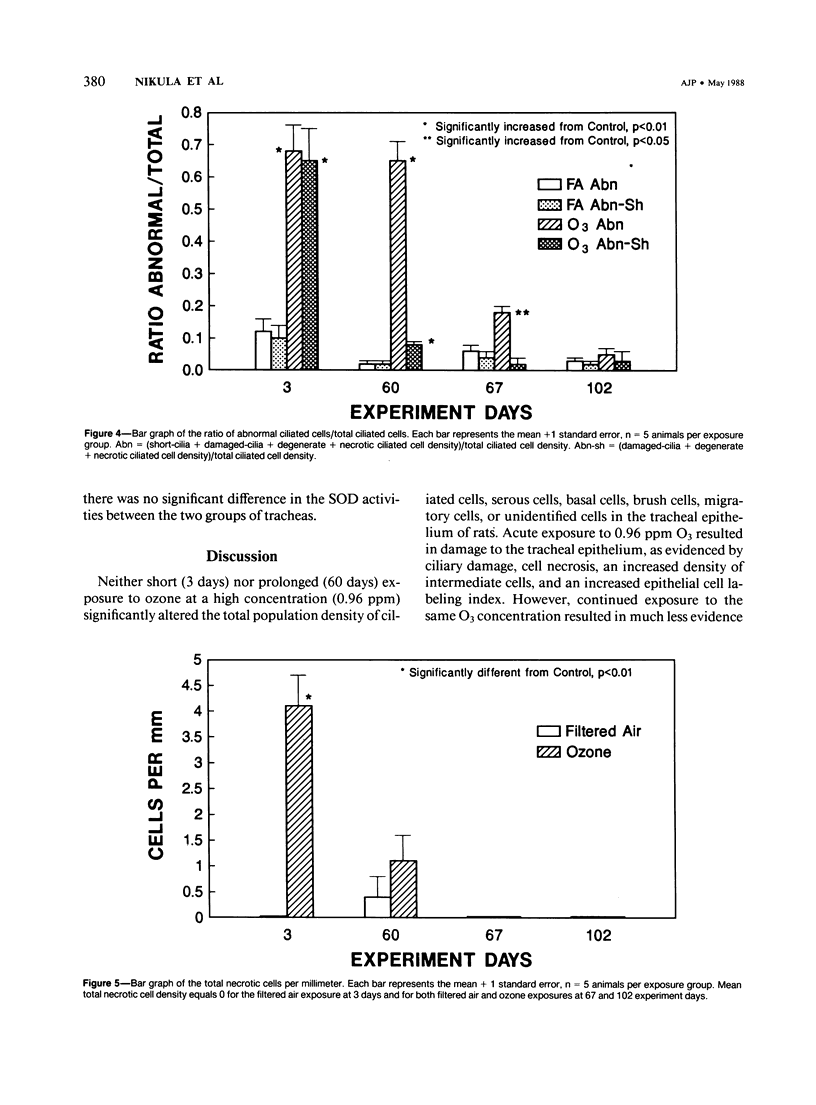
Although acute ozone (O3) exposure injures tracheal epithelium, the response of the tracheal epithelium to prolonged O3 exposure, and the degree of repair following cessation of exposure have not been previously reported. The purpose of this experiment was to characterize the morphologic response of rat tracheal epithelium to acute (3 days) and prolonged (60 days) exposure to 0.96 ppm O3 as well as to evaluate repair in a 7- and 42-day post-60-day exposure period. Quantitative light- and electron-microscopic evaluation and thymidine labeling indices showed that after 3 days of O3 exposure there was ciliary damage, cell necrosis, an increased density of intermediate cells, and an elevated thymidine labeling index. Following 60 days of exposure, the only major change from controls was the presence of ciliated cells with uniformly short cilia. Tracheal superoxide dismutase levels did not differ between control and 60-day exposure groups. Our findings suggest that the tracheal epithelium adapts to prolonged ozone exposure with the exception of cilia formation in ciliated cells. Complete epithelial recovery occurred by 42 days after exposure.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry B. E., Miller F. J., Crapo J. D. Effects of inhalation of 0.12 and 0.25 parts per million ozone on the proximal alveolar region of juvenile and adult rats. Lab Invest. 1985 Dec;53(6):692–704. [PubMed] [Google Scholar]
- Bindreiter M., Schuppler J., Stockinger L. Zellproliferation und Differenzierung im Trachealepithel der Ratte. Exp Cell Res. 1968 May;50(2):377–382. [PubMed] [Google Scholar]
- Boatman E. S., Sato S., Frank R. Acute effects of ozone on cat lungs. II. Structural. Am Rev Respir Dis. 1974 Aug;110(2):157–169. doi: 10.1164/arrd.1974.110.2.157. [DOI] [PubMed] [Google Scholar]
- Boorman G. A., Schwartz L. W., Dungworth D. L. Pulmonary effects of prolonged ozone insult in rats. Morphometric evaluation of the central acinus. Lab Invest. 1980 Aug;43(2):108–115. [PubMed] [Google Scholar]
- Breeze R. G., Wheeldon E. B. The cells of the pulmonary airways. Am Rev Respir Dis. 1977 Oct;116(4):705–777. doi: 10.1164/arrd.1977.116.4.705. [DOI] [PubMed] [Google Scholar]
- Castleman W. L., Dungworth D. L., Schwartz L. W., Tyler W. S. Acute respiratory bronchiolitis: an ultrastructural and autoradiographic study of epithelial cell injury and renewal in rhesus monkeys exposed to ozone. Am J Pathol. 1980 Mar;98(3):811–840. [PMC free article] [PubMed] [Google Scholar]
- Castleman W. L., Tyler W. S., Dungworth D. L. Lesions in respiratory bronchioles and conducting airways of monkeys exposed to ambient levels of ozone. Exp Mol Pathol. 1977 Jun;26(3):384–400. doi: 10.1016/0014-4800(77)90041-7. [DOI] [PubMed] [Google Scholar]
- Chow C. K., Plopper C. G., Dungworth D. L. Influence of dietary vitamin E on the lungs of ozone-exposed rats: a correlated biochemical and histological study. Environ Res. 1979 Dec;20(2):309–317. doi: 10.1016/0013-9351(79)90006-9. [DOI] [PubMed] [Google Scholar]
- Chow C. K., Tappel A. L. An enzymatic protective mechanism against lipid peroxidation damage to lungs of ozone-exposed rats. Lipids. 1972 Aug;7(8):518–524. doi: 10.1007/BF02533017. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Foscue H. A., Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980 Jul;122(1):123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- DeLucia A. J., Hoque P. M., Mustafa M. G., Cross C. E. Ozone interaction with rodent lung: effect on sulfhydryls and sulfhydryl-containing enzyme activities. J Lab Clin Med. 1972 Oct;80(4):559–566. [PubMed] [Google Scholar]
- Dubick M. A., Keen C. L. Tissue trace elements and lung superoxide dismutase activity in mice exposed to ozone. Toxicol Lett. 1983 Jul;17(3-4):355–360. doi: 10.1016/0378-4274(83)90250-3. [DOI] [PubMed] [Google Scholar]
- Elsayed N. M., Mustafa M. G., Postlethwait E. M. Age-dependent pulmonary response of rats to ozone exposure. J Toxicol Environ Health. 1982 May-Jun;9(5-6):835–848. doi: 10.1080/15287398209530206. [DOI] [PubMed] [Google Scholar]
- Eustis S. L., Schwartz L. W., Kosch P. C., Dungworth D. L. Chronic bronchiolitis in nonhuman primates after prolonged ozone exposure. Am J Pathol. 1981 Nov;105(2):121–137. [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Cabral-Anderson L. J., Freeman G. Role of the Clara cell in renewal of the bronchiolar epithelium. Lab Invest. 1978 Jun;38(6):648–653. [PubMed] [Google Scholar]
- Evans M. J., Dekker N. P., Cabral-Anderson L. J., Shami S. G. Morphological basis of tolerance to ozone. Exp Mol Pathol. 1985 Jun;42(3):366–376. doi: 10.1016/0014-4800(85)90086-3. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Johnson L. V., Stephens R. J., Freeman G. Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Lab Invest. 1976 Sep;35(3):246–257. [PubMed] [Google Scholar]
- Evans M. J., Shami S. G., Cabral-Anderson L. J., Dekker N. P. Role of nonciliated cells in renewal of the bronchial epithelium of rats exposed to NO2. Am J Pathol. 1986 Apr;123(1):126–133. [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Sornberger G. C., Huber G. L. A morphometric analysis of the male and female tracheal epithelium after experimental exposure to marijuana smoke. Lab Invest. 1980 Jan;42(1):65–69. [PubMed] [Google Scholar]
- Hayashi M., Sornberger G. C., Huber G. L. Differential response in the male and female tracheal epithelium following exposure to tobacco smoke. Chest. 1978 Apr;73(4):515–518. doi: 10.1378/chest.73.4.515. [DOI] [PubMed] [Google Scholar]
- Jackson R. M., Frank L. Ozone-induced tolerance to hyperoxia in rats. Am Rev Respir Dis. 1984 Mar;129(3):425–429. doi: 10.1164/arrd.1984.129.3.425. [DOI] [PubMed] [Google Scholar]
- Jeffery P. K., Reid L. New observations of rat airway epithelium: a quantitative and electron microscopic study. J Anat. 1975 Nov;120(Pt 2):295–320. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamb D., Reid L. Mitotic rates, goblet cell increase and histochemical changes in mucus in rat bronchial epithelium during exposure to sulphur dioxide. J Pathol Bacteriol. 1968 Jul;96(1):97–111. doi: 10.1002/path.1700960111. [DOI] [PubMed] [Google Scholar]
- Last J. A., Gerriets J. E., Hyde D. M. Synergistic effects on rat lungs of mixtures of oxidant air pollutants (ozone or nitrogen dioxide) and respirable aerosols. Am Rev Respir Dis. 1983 Sep;128(3):539–544. doi: 10.1164/arrd.1983.128.3.539. [DOI] [PubMed] [Google Scholar]
- Lum H., Schwartz L. W., Dungworth D. L., Tyler W. S. A comparative study of cell renewal after exposure to ozone or oxygen. Response of terminal bronchiolar epithelium in the rat. Am Rev Respir Dis. 1978 Aug;118(2):335–345. doi: 10.1164/arrd.1978.118.2.335. [DOI] [PubMed] [Google Scholar]
- Mellick P. W., Dungworth D. L., Schwartz L. W., Tyler W. S. Short term morphologic effects of high ambient levels of ozone on lungs of rhesus monkeys. Lab Invest. 1977 Jan;36(1):82–90. [PubMed] [Google Scholar]
- Menzel D. B. Ozone: an overview of its toxicity in man and animals. J Toxicol Environ Health. 1984;13(2-3):183–204. [PubMed] [Google Scholar]
- Menzel D. B. Toxicity of ozone, oxygen, and radiation. Annu Rev Pharmacol. 1970;10:379–394. doi: 10.1146/annurev.pa.10.040170.002115. [DOI] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972 May 25;247(10):3170–3175. [PubMed] [Google Scholar]
- Moore P. F., Schwartz L. W. Morphological effects of prolonged exposure to ozone and sulfuric acid aerosol on the rat lung. Exp Mol Pathol. 1981 Aug;35(1):108–123. doi: 10.1016/0014-4800(81)90011-3. [DOI] [PubMed] [Google Scholar]
- Mustafa M. G., Elsayed N. M., Quinn C. L., Postlethwait E. M., Gardner D. E., Graham J. A. Comparison of pulmonary biochemical effects of low-level ozone exposure on mice and rats. J Toxicol Environ Health. 1982 May-Jun;9(5-6):857–865. doi: 10.1080/15287398209530208. [DOI] [PubMed] [Google Scholar]
- Pearsall A. D., Echt R., Ross L. M., Roth R. A., Jr, Dinerstein R. J. Morphologic and cytochemical characteristics of amine-containing globule leukocytes in rat tracheal epithelium. Am J Anat. 1984 May;170(1):83–99. doi: 10.1002/aja.1001700107. [DOI] [PubMed] [Google Scholar]
- Penha P. D., Werthamer S. Pulmonary lesions induced by long-term exposure to ozone. II. Ultrastructure observations of proliferative and regressive lesions. Arch Environ Health. 1974 Nov;29(5):282–289. doi: 10.1080/00039896.1974.10666588. [DOI] [PubMed] [Google Scholar]
- Plopper C. G., Chow C. K., Dungworth D. L., Brummer M., Nemeth T. J. Effect of low level of ozone on rat lungs. II. Morphological responses during recovery and re-exposure. Exp Mol Pathol. 1978 Dec;29(3):400–411. doi: 10.1016/0014-4800(78)90081-3. [DOI] [PubMed] [Google Scholar]
- Plopper C. G., Mariassy A. T., Wilson D. W., Alley J. L., Nishio S. J., Nettesheim P. Comparison of nonciliated tracheal epithelial cells in six mammalian species: ultrastructure and population densities. Exp Lung Res. 1983 Dec;5(4):281–294. doi: 10.3109/01902148309061521. [DOI] [PubMed] [Google Scholar]
- Reasor M. J., Adams G. K., 3rd, Brooks J. K., Rubin R. J. Enrichment of albumin and IgG in the airway secretions of dogs breathing ozone. J Environ Sci Health C. 1979;13(4):335–346. [PubMed] [Google Scholar]
- SPICER S. S. DIAMINE METHODS FOR DIFFERENTIALING MUCOSUBSTANCES HISTOCHEMICALLY. J Histochem Cytochem. 1965 Mar;13:211–234. doi: 10.1177/13.3.211. [DOI] [PubMed] [Google Scholar]
- Schwartz L. W., Dungworth D. L., Mustafa M. G., Tarkington B. K., Tyler W. S. Pulmonary responses of rats to ambient levels of ozone: effects of 7-day intermittent or continuous exposure. Lab Invest. 1976 Jun;34(6):565–578. [PubMed] [Google Scholar]
- Wells A. B. The kinetics of cell proliferation in the tracheobronchial epithelia of rats with and without chronic respiratory disease. Cell Tissue Kinet. 1970 Apr;3(2):185–206. doi: 10.1111/j.1365-2184.1970.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Wilson D. W., Plopper C. G., Dungworth D. L. The response of the macaque tracheobronchial epithelium to acute ozone injury. A quantitative ultrastructural and autoradiographic study. Am J Pathol. 1984 Aug;116(2):193–206. [PMC free article] [PubMed] [Google Scholar]